Redox Regulation of PTEN by Reactive Oxygen Species: Its Role in Physiological Processes
- PMID: 38397797
- PMCID: PMC10886030
- DOI: 10.3390/antiox13020199
Redox Regulation of PTEN by Reactive Oxygen Species: Its Role in Physiological Processes
Abstract
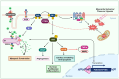
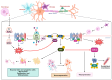
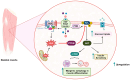
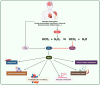
Phosphatase and tensin homolog (PTEN) is a tumor suppressor due to its ability to regulate cell survival, growth, and proliferation by downregulating the PI3K/AKT signaling pathway. In addition, PTEN plays an essential role in other physiological events associated with cell growth demands, such as ischemia-reperfusion, nerve injury, and immune responsiveness. Therefore, recently, PTEN inhibition has emerged as a potential therapeutic intervention in these situations. Increasing evidence demonstrates that reactive oxygen species (ROS), especially hydrogen peroxide (H2O2), are produced and required for the signaling in many important cellular processes under such physiological conditions. ROS have been shown to oxidize PTEN at the cysteine residue of its active site, consequently inhibiting its function. Herein, we provide an overview of studies that highlight the role of the oxidative inhibition of PTEN in physiological processes.
Keywords: PTEN; ROS; cell signaling; oxidative inhibition; redox regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Redox Regulation of Phosphatase and Tensin Homolog by Bicarbonate and Hydrogen Peroxide: Implication of Peroxymonocarbonate in Cell Signaling.Antioxidants (Basel). 2024 Apr 17;13(4):473. doi: 10.3390/antiox13040473. Antioxidants (Basel). 2024. PMID: 38671920 Free PMC article.
-
Redox Regulation of PTEN by Peroxiredoxins.Antioxidants (Basel). 2021 Feb 16;10(2):302. doi: 10.3390/antiox10020302. Antioxidants (Basel). 2021. PMID: 33669370 Free PMC article. Review.
-
Intracellular redox status controls membrane localization of pro- and anti-migratory signaling molecules.Redox Biol. 2014 Jan 20;2:245-50. doi: 10.1016/j.redox.2014.01.005. eCollection 2014. Redox Biol. 2014. PMID: 24494199 Free PMC article.
-
PTEN promotes apoptosis of H2O2‑injured rat nasal epithelial cells through PI3K/Akt and other pathways.Mol Med Rep. 2018 Jan;17(1):571-579. doi: 10.3892/mmr.2017.7912. Epub 2017 Oct 26. Mol Med Rep. 2018. PMID: 29115519
-
Redox regulation of tumor suppressor PTEN in cell signaling.Redox Biol. 2020 Jul;34:101553. doi: 10.1016/j.redox.2020.101553. Epub 2020 May 3. Redox Biol. 2020. PMID: 32413744 Free PMC article. Review.
Cited by
-
Systematic and comprehensive insights into HIF-1 stabilization under normoxic conditions: implications for cellular adaptation and therapeutic strategies in cancer.Cell Mol Biol Lett. 2025 Jan 6;30(1):2. doi: 10.1186/s11658-024-00682-7. Cell Mol Biol Lett. 2025. PMID: 39757165 Free PMC article. Review.
-
The Importance of Phosphoinositide 3-Kinase in Neuroinflammation.Int J Mol Sci. 2024 Oct 30;25(21):11638. doi: 10.3390/ijms252111638. Int J Mol Sci. 2024. PMID: 39519189 Free PMC article. Review.
-
The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective.Nutrients. 2025 Jul 18;17(14):2354. doi: 10.3390/nu17142354. Nutrients. 2025. PMID: 40732979 Free PMC article. Review.
-
Redox Regulation of Phosphatase and Tensin Homolog by Bicarbonate and Hydrogen Peroxide: Implication of Peroxymonocarbonate in Cell Signaling.Antioxidants (Basel). 2024 Apr 17;13(4):473. doi: 10.3390/antiox13040473. Antioxidants (Basel). 2024. PMID: 38671920 Free PMC article.
-
Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling.Int J Mol Sci. 2024 Jun 1;25(11):6114. doi: 10.3390/ijms25116114. Int J Mol Sci. 2024. PMID: 38892300 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

