Redox Biomarkers and Matrix Remodeling Molecules in Ovarian Cancer
- PMID: 38397798
- PMCID: PMC10885995
- DOI: 10.3390/antiox13020200
Redox Biomarkers and Matrix Remodeling Molecules in Ovarian Cancer
Abstract
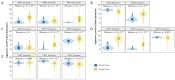
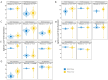
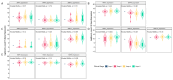
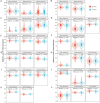
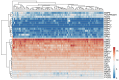
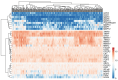
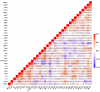
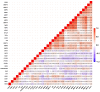
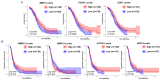
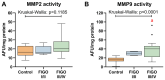
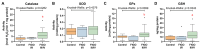
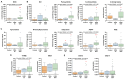
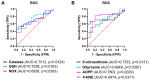
Ovarian cancer (OC) has emerged as the leading cause of death due to gynecological malignancies among women. Oxidative stress and metalloproteinases (MMPs) have been shown to influence signaling pathways and afflict the progression of carcinogenesis. Therefore, the assessment of matrix-remodeling and oxidative stress intensity can determine the degree of cellular injury and often the severity of redox-mediated chemoresistance. The study group comprised 27 patients with serous OC of which 18% were classified as Federation of Gynecology and Obstetrics (FIGO) stages I/II, while the rest were diagnosed grades III/IV. The control group comprised of 15 ovarian tissue samples. The results were compared with genetic data from The Cancer Genome Atlas. Nitro-oxidative stress, inflammation and apoptosis biomarkers were measured colorimetrically/fluorometrically or via real-time PCR in the primary ovarian tumor and healthy tissue. Stratification of patients according to FIGO stages revealed that high-grade carcinoma exhibited substantial alterations in redox balance, including the accumulation of protein glycoxidation and lipid peroxidation products. TCGA data demonstrated only limited prognostic usefulness of the studied genes. In conclusion, high-grade serous OC is associated with enhanced tissue oxidative/nitrosative stress and macromolecule damage that could not be overridden by the simultaneously augmented measures of antioxidant defense. Therefore, it can be assumed that tumor cells acquire adaptive mechanisms that enable them to withstand the potential toxic effects of elevated reactive oxygen species.
Keywords: apoptosis; inflammation; nitrosative stress; ovarian neoplasms; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Could circulating biomarkers of nitrosative stress and protein glycoxidation be useful in patients with gastric cancer?Front Oncol. 2023 Jul 12;13:1213802. doi: 10.3389/fonc.2023.1213802. eCollection 2023. Front Oncol. 2023. PMID: 37503318 Free PMC article.
-
Preoperative serum 8-hydroxydeoxyguanosine is associated with chemoresistance and is a powerful prognostic factor in endometrioid-type epithelial ovarian cancer.BMC Cancer. 2015 Jul 2;15:493. doi: 10.1186/s12885-015-1504-6. BMC Cancer. 2015. PMID: 26134400 Free PMC article.
-
[Current FIGO staging classification for cancer of ovary, fallopian tube and peritoneum].Ceska Gynekol. 2017 Summer;82(3):230-236. Ceska Gynekol. 2017. PMID: 28593778 Review. Czech.
-
VAV1 represses E-cadherin expression through the transactivation of Snail and Slug: a potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer.Transl Res. 2013 Sep;162(3):181-90. doi: 10.1016/j.trsl.2013.06.005. Epub 2013 Jul 12. Transl Res. 2013. PMID: 23856093
-
New Achievements from Molecular Biology and Treatment Options for Refractory/Relapsed Ovarian Cancer-A Systematic Review.Cancers (Basel). 2023 Nov 10;15(22):5356. doi: 10.3390/cancers15225356. Cancers (Basel). 2023. PMID: 38001616 Free PMC article. Review.
Cited by
-
Reactive oxygen and nitrogen species: multifaceted regulators of ovarian activity†.Biol Reprod. 2025 May 13;112(5):789-806. doi: 10.1093/biolre/ioaf032. Biol Reprod. 2025. PMID: 39936599 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

