Conjecturing about Small-Molecule Agonists and Antagonists of α4β1 Integrin: From Mechanistic Insight to Potential Therapeutic Applications
- PMID: 38397918
- PMCID: PMC10887150
- DOI: 10.3390/biomedicines12020316
Conjecturing about Small-Molecule Agonists and Antagonists of α4β1 Integrin: From Mechanistic Insight to Potential Therapeutic Applications
Abstract
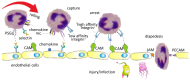
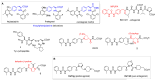
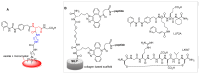
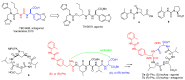
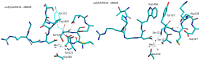
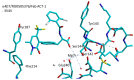
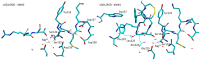
Integrins are heterodimeric cell-surface receptors that regulate cell-cell adhesion and cellular functions through bidirectional signaling. On the other hand, anomalous trafficking of integrins is also implicated in severe pathologies as cancer, thrombosis, inflammation, allergies, and multiple sclerosis. For this reason, they are attractive candidates as drug targets. However, despite promising preclinical data, several anti-integrin drugs failed in late-stage clinical trials for chronic indications, with paradoxical side effects. One possible reason is that, at low concentration, ligands proposed as antagonists may also act as partial agonists. Hence, the comprehension of the specific structural features for ligands' agonism or antagonism is currently of the utmost interest. For α4β1 integrin, the situation is particularly obscure because neither the crystallographic nor the cryo-EM structures are known. In addition, very few potent and selective agonists are available for investigating the mechanism at the basis of the receptor activation. In this account, we discuss the physiological role of α4β1 integrin and the related pathologies, and review the few agonists. Finally, we speculate on plausible models to explain agonism vs. antagonism by comparison with RGD-binding integrins and by analysis of computational simulations performed with homology or hybrid receptor structures.
Keywords: agonist; crystal structure; inflammation; molecular docking; α4β1 integrin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
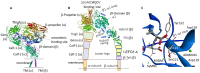
Similar articles
-
Design and Pharmacological Characterization of α4β1 Integrin Cyclopeptide Agonists: Computational Investigation of Ligand Determinants for Agonism versus Antagonism.J Med Chem. 2023 Apr 13;66(7):5021-5040. doi: 10.1021/acs.jmedchem.2c02098. Epub 2023 Mar 28. J Med Chem. 2023. PMID: 36976921 Free PMC article.
-
Design, Pharmacological Characterization, and Molecular Docking of Minimalist Peptidomimetic Antagonists of α4β1 Integrin.Int J Mol Sci. 2023 May 31;24(11):9588. doi: 10.3390/ijms24119588. Int J Mol Sci. 2023. PMID: 37298541 Free PMC article.
-
An Overview of the α4β1 Integrin and the Potential Therapeutic Role of its Antagonists.Curr Med Chem. 2021;28(29):5884-5895. doi: 10.2174/0929867328666210217153609. Curr Med Chem. 2021. PMID: 33596793 Review.
-
A general chemical principle for creating closure-stabilizing integrin inhibitors.Cell. 2022 Sep 15;185(19):3533-3550.e27. doi: 10.1016/j.cell.2022.08.008. Cell. 2022. PMID: 36113427 Free PMC article.
-
Novel Ligands Targeting α4β1 Integrin: Therapeutic Applications and Perspectives.Front Chem. 2019 Jul 9;7:489. doi: 10.3389/fchem.2019.00489. eCollection 2019. Front Chem. 2019. PMID: 31338363 Free PMC article. Review.
Cited by
-
Development of VLA4 and CXCR4 Antagonists for the Mobilization of Hematopoietic Stem and Progenitor Cells.Biomolecules. 2024 Aug 14;14(8):1003. doi: 10.3390/biom14081003. Biomolecules. 2024. PMID: 39199390 Free PMC article. Review.
-
GA-LDV: A Promising Derivative of 18β-Glycyrrhetinic Acid with Enhanced in vitro and in vivo Anti-Cancer Properties.Drug Des Devel Ther. 2025 Apr 4;19:2641-2652. doi: 10.2147/DDDT.S492303. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40201197 Free PMC article.
-
Integrin Alpha8 Beta1 (81): An In-Depth Review of an Overlooked RGD-Binding Receptor.Biocell. 2025;49(5):789-811. doi: 10.32604/biocell.2025.062325. Epub 2025 May 27. Biocell. 2025. PMID: 40510035 Free PMC article.
References
-
- Cabodi S., Di Stefano P., del Pilar Camacho Leal M., Tinnirello A., Bisaro B., Morello V., Damiano L., Aramu S., Repetto D., Tornillo G., et al. Integrins and signal transduction. Adv. Exp. Med. Biol. 2010;674:43–54. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

