Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy
- PMID: 38397988
- PMCID: PMC10886574
- DOI: 10.3390/biomedicines12020386
Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy
Abstract
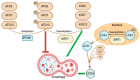
Sirtuins (SIRTs) are stress-responsive proteins that regulate several post-translational modifications, partly by acetylation, deacetylation, and affecting DNA methylation. As a result, they significantly regulate several cellular processes. In essence, they prolong lifespan and control the occurrence of spontaneous tumor growth. Members of the SIRT family have the ability to govern embryonic, hematopoietic, and other adult stem cells in certain tissues and cell types in distinct ways. Likewise, they can have both pro-tumor and anti-tumor effects on cancer stem cells, contingent upon the specific tissue from which they originate. The impact of autophagy on cancer stem cells, which varies depending on the specific circumstances, is a very intricate phenomenon that has significant significance for clinical and therapeutic purposes. SIRTs exert an impact on the autophagy process, whereas autophagy reciprocally affects the activity of certain SIRTs. The mechanism behind this connection in cancer stem cells remains poorly understood. This review presents the latest findings that position SIRTs at the point where cancer cells and autophagy interact. Our objective is to highlight the various roles of distinct SIRTs in cancer stem cell-related functions through autophagy. This would demonstrate their significance in the genesis and recurrence of cancer and offer a more precise understanding of their treatment possibilities in relation to autophagy.
Keywords: DNA methylation; SIRT; acetylation; autophagy; cancer stem cells; deacetylation; epigenetics; sirtuins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Role of Sirtuins in Maintenance of Genomic Stability: Relevance to Cancer and Healthy Aging.DNA Cell Biol. 2016 Oct;35(10):542-575. doi: 10.1089/dna.2016.3280. Epub 2016 Jul 5. DNA Cell Biol. 2016. PMID: 27380140 Review.
-
Emerging role of sirtuins in non‑small cell lung cancer (Review).Oncol Rep. 2024 Oct;52(4):127. doi: 10.3892/or.2024.8786. Epub 2024 Aug 2. Oncol Rep. 2024. PMID: 39092574 Free PMC article. Review.
-
Sirtuins at the crossroads of stemness, aging, and cancer.Aging Cell. 2017 Dec;16(6):1208-1218. doi: 10.1111/acel.12685. Epub 2017 Oct 10. Aging Cell. 2017. PMID: 28994177 Free PMC article. Review.
-
Role of sirtuins in sepsis and sepsis-induced organ dysfunction: A review.Int J Biol Macromol. 2024 Oct;278(Pt 3):134853. doi: 10.1016/j.ijbiomac.2024.134853. Epub 2024 Aug 19. Int J Biol Macromol. 2024. PMID: 39163955 Review.
-
Sirtuin Family Members Selectively Regulate Autophagy in Osteosarcoma and Mesothelioma Cells in Response to Cellular Stress.Front Oncol. 2019 Sep 24;9:949. doi: 10.3389/fonc.2019.00949. eCollection 2019. Front Oncol. 2019. PMID: 31608237 Free PMC article.
Cited by
-
Relationship between Serum Sirtuin 1 and Growth Hormone/Insulin-like Growth Factor 1 Concentrations in Children with Growth Hormone Deficiency and Idiopathic Short Stature.Biomedicines. 2024 Jun 27;12(7):1433. doi: 10.3390/biomedicines12071433. Biomedicines. 2024. PMID: 39062007 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

