Interleukin-6 as a Predictive Factor of Pathological Response to FLOT Regimen Systemic Treatment in Locally Advanced Gastroesophageal Junction or Gastric Cancer Patients
- PMID: 38398148
- PMCID: PMC10887209
- DOI: 10.3390/cancers16040757
Interleukin-6 as a Predictive Factor of Pathological Response to FLOT Regimen Systemic Treatment in Locally Advanced Gastroesophageal Junction or Gastric Cancer Patients
Abstract
Background: Perioperative treatment is a gold standard in locally advanced gastric cancer or GEJ cancer in the Western population. Unfortunately, the response rate after neoadjuvant chemotherapy (NAC) remains limited. Moreover, there are currently no biomarkers enabling an individual prediction of therapeutic efficacy. The aim of this study was the identification of serum biomarkers of early response to NAC.
Methods: We conducted this prospective study in the MSCNRIO in Warsaw, Poland. A total of 71 patients and 15 healthy volunteers gave informed consent. Complete blood count, carcinoembryonic antigen (CEA), carcinoma antigen 125 (CA125), carcinoma antigen 19.9 (CA19.9), and fibrinogen (F) were measured at baseline and before every cycle. Circulating tumour cells (CTCs) and interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-10 (IL-10) were measured in a pilot group of 40 patients at baseline and before cycle two (C2) and cycle three (C3).
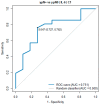
Results: Of all the measured parameters, only the IL-6 serum level was statistically significant. The IL-6 level before C2 of chemotherapy was significantly decreased in the complete pathological response (pCR) vs. the non-pCR group (3.71 pg/mL vs. 7.63 pg/mL, p = 0.004). In all patients with an IL-6 level below 5.0 pg/mL in C2, tumour regression TRG1a/1b according to the Becker classification and ypN0 were detected in postoperative histopathological specimens. The IL-6 level before C1 of chemotherapy was significantly elevated in ypN+ vs. ypN0 (7.69 pg/mL vs. 2.89 pg/mL, p = 0.022).
Conclusions: The trial showed that an elevated level of IL-6 prior to treatment and C2 might be a predictor of pathological response to NAC.
Keywords: FLOT regimen; IL-6; gastric cancer; gastroesophageal junction cancer; predictive biomarker.
Conflict of interest statement
Katarzyna Marcisz-Grzanka, Beata Kotowicz, Aleksandra Nowak, Mariola Winiarek, Malgorzata Fuksiewicz, Maria Kowalska, Andrzej Tysarowski, Tomasz Olesinski, Jakub Palucki, Urszula Sulkowska, and Agnieszka Kolasinska-Cwikla declare no conflicts of interest. Lucjan Stanislaw Wyrwicz: consulting fees—MSD, AstraZeneca; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events—MSD, AstraZeneca; payment for expert testimony—MSD, AstraZeneca.
Figures




Similar articles
-
Perioperative treatment in resectable gastric cancer with spartalizumab in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT): a phase II study (GASPAR).BMC Cancer. 2022 May 12;22(1):537. doi: 10.1186/s12885-022-09623-z. BMC Cancer. 2022. PMID: 35549674 Free PMC article. Clinical Trial.
-
Safety and efficacy of the FLOT regimen in the Polish population - an analysis of the prospective trial.Neoplasma. 2022 Dec;69(6):1445-1450. doi: 10.4149/neo_2022_220720N734. Epub 2022 Nov 11. Neoplasma. 2022. PMID: 36353936 Clinical Trial.
-
Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial.Lancet Oncol. 2016 Dec;17(12):1697-1708. doi: 10.1016/S1470-2045(16)30531-9. Epub 2016 Oct 22. Lancet Oncol. 2016. PMID: 27776843 Clinical Trial.
-
FLOT or CROSS for gastroesophageal junction cancers-is the debate over yet?Chin Clin Oncol. 2023 Jun;12(3):24. doi: 10.21037/cco-23-5. Epub 2023 May 25. Chin Clin Oncol. 2023. PMID: 37303220 Review.
-
[Does the FLOT regimen a new standard of perioperative chemotherapy for localized gastric cancer?].Bull Cancer. 2020 Jan;107(1):54-60. doi: 10.1016/j.bulcan.2019.12.005. Epub 2020 Jan 21. Bull Cancer. 2020. PMID: 31980145 Review. French.
Cited by
-
Integrating Network Pharmacology and In Silico Analysis to Explore the Bioactive Compounds Against Gastric Cancer Treatment.Cureus. 2024 Dec 16;16(12):e75779. doi: 10.7759/cureus.75779. eCollection 2024 Dec. Cureus. 2024. PMID: 39816318 Free PMC article.
-
A Multi-marker model based on serum IL-10 predicts response to conversion immunochemotherapy in gastric cancer patients: a retrospective cohort study.BMC Gastroenterol. 2025 Aug 27;25(1):621. doi: 10.1186/s12876-025-04136-y. BMC Gastroenterol. 2025. PMID: 40866830 Free PMC article.
References
-
- Chau I., Norman A.R., Cunningham D., Waters J.S., Oates J., Ross P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. - DOI - PubMed
-
- Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M., Scarffe J.H., Lofts F.J., Falk S.J., Iveson T.J., et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
-
- Ychou M., Boige V., Pignon J.P., Conroy T., Bouche O., Lebreton G., Ducourtieux M., Bedenne L., Fabre J.M., Saint-Aubert B., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011;1715:21444866. doi: 10.1200/JCO.2010.33.0597. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

