An Update on Current Therapeutic Options in IgA Nephropathy
- PMID: 38398259
- PMCID: PMC10889409
- DOI: 10.3390/jcm13040947
An Update on Current Therapeutic Options in IgA Nephropathy
Abstract
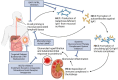
Immunoglobulin A nephropathy (IgAN) remains the leading cause of primary glomerular disease worldwide. Outcomes are poor with high rates of progressive chronic kidney disease and kidney failure, which contributes to global healthcare costs. Although this disease entity has been described, there were no disease-specific treatments until recently, with the current standard of care focusing on optimal supportive measures including lifestyle modifications and optimization of the renin-angiotensin-aldosterone blockade. However, with significant advances in the understanding of the pathogenesis of IgAN in the past decade, and the acceptance of surrogate outcomes for accelerated drug approval, there have been many new investigational agents tested to target this disease. As these agents become available, we envision a multi-pronged treatment strategy that simultaneously targets the consequences of ongoing nephron loss, stopping any glomerular inflammation, inhibiting pro-fibrotic signals in the glomerulus and tubulo-interstitium, and inhibiting the production of pathogenic IgA molecules. This review is an update on a previous review published in 2021, and we aim to summarize the developments and updates in therapeutic strategies in IgAN and highlight the promising discoveries that are likely to add to our armamentarium.
Keywords: IgA; IgA nephropathy; clinical trials; glomerular diseases; therapy; treatment.
Conflict of interest statement
R.S.L. and S.C.Y. have no conflict of interest to declare. J.B. has received research grants from Argenx, Calliditas Therapeutics, Chinook Therapeutics, Galapagos NV, GlaxoSmithKline, Novartis and Travere Therapeutics, and is medical/scientific advisor to Alnylam Pharmaceuticals, Argenx, Astellas Pharma, BioCryst Pharmaceuticals, Calliditas Therapeutics, Chinook Therapeutics, Dimerix, Galapagos NV, Novartis, Omeros, Travere Therapeutics, UCB, Vera Therapeutics and Visterra. D.V.R. has received grant funding from Achilion Pharmaceuticals Inc., Reata Pharmaceuticals, Calliditas Therapeutics, Travere Therapeutics, Pfizer Inc., and Morphosys Pharmaceuticals. D.V.R. has received personal fees from Visterra, Novartis and Otsuka Pharmaceuticals, and holds equity in Reliant Glycosciences LLC.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

