Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up
- PMID: 38398401
- PMCID: PMC10889517
- DOI: 10.3390/jcm13041088
Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up
Abstract
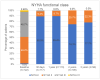
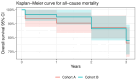
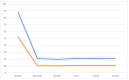
Aims: We report 30-day, 1-year, and 3-year outcomes for a new TAVR programme that used five different transcatheter heart valve (THV) systems. Methods: From 2014 to 2020, 122 consecutive patients with severe aortic stenosis (AS) received TAVR based on the Heart Team decision. Outcomes were analysed for the whole study population and in addition the first 63 patients (Cohort A, 2014 to 2019) were compared to the last 59 patients (Cohort B, 2019 to 2020). Outcomes included VARC-2 definitions and device performance assessed via transthoracic echocardiography by independent high-volume investigators. Results: The mean patient age was 77.9 ± 6.1 years old, and 48 (39.3%) were male. The mean logistic Euroscore II was 4.2 ± 4.5, and the mean STS score was 6.9 ± 4.68. The systems used were as follows: Medtronic Corevalve Evolute R/PRO (82 patients-67.2%); Abbott Portico (13-10.6%); Boston Scientific Lotus (10-8.2%); Meril Myval (11-9%); and Boston Scientific Neo Accurate (6-5%). Access was transfemoral (95.9% of patients); surgical cut down (18% vs. percutaneous 77.8%); subclavian (n = 2); trans-axillary (n = 2); and direct aorta (n = 1). VARC-2 outcomes were as follows: device success rate 97.5%; stroke rate 1.6%; major vascular complication 3.3%; permanent pacemaker implantation 12.4%. At discharge, the incidences of grade I and II aortic regurgitation were 39.95 and 55.5%, respectively. At one year, all-cause mortality was 7.4% without admissions for valve-related dysfunction. The 3-year all-cause mortality and all-stroke rates were 22.9% and 4.1%, respectively. Between the 1-year and 3-year follow-ups, valve-related dysfunction was detected in three patients; one had THV system endocarditis that led to death. There was a remarkable but statistically non-significant decrease in mortality from Cohort A to Cohort B [four (6.3%) vs. one patient (1.7%), p = 0.195] and major vascular complications occurred at a significantly higher rate in the Cohort B [zero (0%) vs. four (6.8% patient, p = 0.036)]. Overall, we found that using multiple devices was safe and allowed for a learning team to achieve a high device success rate from the beginning (97.5%). Conclusions: TAVR with different THV systems showed acceptable early and mid-term outcomes for survival, technical success, and valve-related adverse events in high-risk patients with significant AS, even in the learning curve phase.
Keywords: TAVR; balloon-expandable transcatheter heart valve; bicuspid aortic valve and radial paradox; learning curve; paravalvular leak; self-expandable transcatheter heart valve.
Conflict of interest statement
The authors have no conflict of interest associated with this publication. There was no financial support for this work that would have influenced its outcome.
Figures



References
-
- Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. - DOI - PubMed
-
- Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Barón-Esquivias G., Baumgartner H., Borger M.A., Carrel T.P., De Bonis M., Evangelista A., et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. J. Cardiothorac. Surg. 2012;42:S1–S44. - PubMed
-
- Forrest J.K., Deeb G.M., Yakubov S.J., Gada H., Mumtaz M.A., Ramlawi B., Bajwa T., Teirstein P.S., DeFrain M., Muppala M., et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2023;81:1663–1674. doi: 10.1016/j.jacc.2023.02.017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

