Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review
- PMID: 38398450
- PMCID: PMC10889526
- DOI: 10.3390/jcm13041136
Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review
Abstract
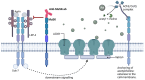
Myasthenia gravis (MG) is a rare autoimmune disease. Transient neonatal myasthenia gravis (TNMG) is caused by pathogenic maternal autoantibodies that cross the placenta and disrupt signaling at the neuromuscular junction. This is a systematic review of this transient immunoglobulin G (IgG)-mediated disease. TNMG affects 10-20% of children born to mothers with MG. The severity of symptoms ranges from minor feeding difficulties to life-threatening respiratory weakness. Minor symptoms might go unnoticed but can still interfere with breastfeeding. Acetylcholine-esterase inhibitors and antibody-clearing therapies such as immunoglobulins can be used to treat TNMG, but most children do well with observation only. TNMG is self-limiting within weeks as circulating antibodies are naturally cleared from the blood. In rare cases, TNMG is associated with permanent skeletal malformations or permanent myopathy. The mother's antibodies can also lead to spontaneous abortions. All healthcare professionals meeting pregnant or birthing women with MG or their neonates should be aware of TNMG. TNMG is hard to predict. Reoccurrence is common among siblings. Pre-pregnancy thymectomy and intravenous immunoglobulins during pregnancy reduce the risk. Neonatal fragment crystallizable receptor (FcRn) blocking drugs for MG might reduce TNMG risk.
Keywords: MuSK antibodies; acetylcholine receptor antibodies; autoantibodies; maternal–fetal exchange; myasthenia gravis; neonatal disease; neuromuscular junction.
Conflict of interest statement
M.H. Bjørk received speaker honoraria and/or served on scientific advisory boards for Teva, Eisai, AbbVie, Pfizer, Novartis, Lundbeck, Angelini Pharma, Jazz pharmaceuticals, and Lilly over the last 5 years. None of the assignments concerned treatment of myasthenia gravis. N.E. Gilhus received financial support from UCB, Argenx, Janssen, Merck, Roche, Alexion, Immunovant, Huma, Denka, Grifols, and Dianthus. J. Lindroos; declare no conflicts of interest.
Figures



Similar articles
-
Transient Neonatal Myasthenia Gravis: A Case Report.Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2023 Jul 15;44(2):165-169. doi: 10.2478/prilozi-2023-0036. Print 2023 Jul 1. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2023. PMID: 37453109
-
The expectant management of a rare neonatal disease: transient neonatal myasthenia gravis.Turk J Pediatr. 2023;65(2):321-325. doi: 10.24953/turkjped.2022.717. Turk J Pediatr. 2023. PMID: 37114697
-
Pregnancy in myasthenia gravis: a retrospective analysis of maternal and neonatal outcome from a large tertiary care centre in Germany.Arch Gynecol Obstet. 2024 Jul;310(1):277-284. doi: 10.1007/s00404-024-07436-y. Epub 2024 Mar 16. Arch Gynecol Obstet. 2024. PMID: 38492082 Free PMC article.
-
Clinical and pathophysiologic relevance of autoantibodies in neonatal myasthenia gravis.Pediatr Neonatol. 2021 Nov;62(6):581-590. doi: 10.1016/j.pedneo.2021.05.020. Epub 2021 Jun 19. Pediatr Neonatol. 2021. PMID: 34272198 Review.
-
Maternal myasthenia gravis represents a risk for the child through autoantibody transfer, immunosuppressive therapy and genetic influence.Eur J Neurol. 2018 Dec;25(12):1402-1409. doi: 10.1111/ene.13788. Epub 2018 Sep 14. Eur J Neurol. 2018. PMID: 30133097 Review.
References
-
- Haddaway N.R., Page M.J., Pritchard C.C., McGuinness L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022;18:e1230. doi: 10.1002/cl2.1230. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

