Establishment and Validation of a Transdermal Drug Delivery System for the Anti-Depressant Drug Citalopram Hydrobromide
- PMID: 38398519
- PMCID: PMC10892536
- DOI: 10.3390/molecules29040767
Establishment and Validation of a Transdermal Drug Delivery System for the Anti-Depressant Drug Citalopram Hydrobromide
Abstract
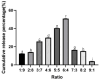
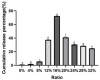
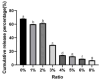
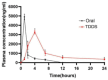
To enhance the bioavailability and antihypertensive effect of the anti-depressant drug citalopram hydrobromide (CTH) we developed a sustained-release transdermal delivery system containing CTH. A transdermal diffusion meter was first used to determine the optimal formulation of the CTH transdermal drug delivery system (TDDS). Then, based on the determined formulation, a sustained-release patch was prepared; its physical characteristics, including quality, stickiness, and appearance, were evaluated, and its pharmacokinetics and irritation to the skin were evaluated by applying it to rabbits and rats. The optimal formulation of the CTH TDDS was 49.2% hydroxypropyl methyl cellulose K100M, 32.8% polyvinylpyrrolidone K30, 16% oleic acid-azone, and 2% polyacrylic acid resin II. The system continuously released an effective dose of CTH for 24 h and significantly enhanced its bioavailability, with a higher area under the curve, good stability, and no skin irritation. The developed CTH TDDS possessed a sustained-release effect and good characteristics and pharmacokinetics; therefore, it has the potential for clinical application as an antidepressant.
Keywords: citalopram hydrobromide; pharmacokinetics; skin irritation; transdermal delivery.
Conflict of interest statement
Authors Zhao Liu and Li-hui Xiao were employed by the Harvest Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Briggs A.M. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

