Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools
- PMID: 38398601
- PMCID: PMC10893115
- DOI: 10.3390/molecules29040849
Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools
Abstract
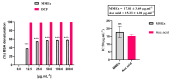
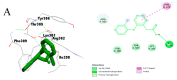
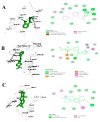
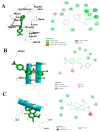
Compounds derived from natural sources continue to serve as chemical scaffolds for designing prophylactic/therapeutic options for human healthcare. In this study, we aimed to systematically unravel the chemical profile and antioxidant and anti-inflammatory activities of myrtle methanolic extract (MMEx) using in vitro, in vivo, and in silico approaches. High levels of TPC (415.85 ± 15.52 mg GAE/g) and TFC (285.80 ± 1.64 mg QE/g) were observed. Mass spectrophotometry (GC-MS) analysis revealed the presence of 1,8-cineole (33.80%), α-pinene (10.06%), linalool (4.83%), p-dimethylaminobenzophenone (4.21%), thunbergol (4%), terpineol (3.60%), cis-geranyl acetate (3.25%), and totarol (3.30%) as major compounds. MMEx induced pronounced dose-dependent inhibition in all assays, and the best antioxidant activity was found with H2O2, with an IC50 of 17.81 ± 3.67 µg.mL-1. MMEx showed a good anti-inflammatory effect in vivo by limiting the development of carrageenan-induced paw edema. The pharmacokinetic profiles of the active molecules were determined using the SwissADME website, followed by virtual screening against anti-inflammatory targets including phospholipase A2 (PLA-2), cyclooxygenase-2 (COX-2), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and NF-κB. A pharmacokinetic study revealed that the molecules have good absorption, distribution, and metabolism profiles, with negative organ toxicity. Among the compounds identified by GC-MS analysis, pinostrobin chalcone, cinnamyl cinnamate, hedycaryol, totarol, and p-dimethylaminobenzophenone were observed to have good binding scores, thus appreciable anti-inflammatory potential. Our study reveals that MMEx from Algerian Myrtus communis L. can be considered to be a promising candidate for alleviating many health complaints associated with oxidative stress and inflammation.
Keywords: ADMET; Myrtus communis L.; anti-inflammatory; antioxidant; bioactive molecules; methanolic extract; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2.Pharmaceuticals (Basel). 2023 Sep 22;16(10):1343. doi: 10.3390/ph16101343. Pharmaceuticals (Basel). 2023. PMID: 37895814 Free PMC article.
-
Phytochemical analysis, bioactivity, and molecular docking studies of Myrtus communis L. seeds and fruit peel extracts demonstrating antioxidant and anti-tyrosinase properties.Sci Rep. 2025 Feb 15;15(1):5634. doi: 10.1038/s41598-025-89401-6. Sci Rep. 2025. PMID: 39955379 Free PMC article.
-
Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower.Food Chem Toxicol. 2010 May;48(5):1362-70. doi: 10.1016/j.fct.2010.03.002. Epub 2010 Mar 6. Food Chem Toxicol. 2010. PMID: 20211674
-
Antioxidant Activity of Myrtus communis L. and Myrtus nivellei Batt. & Trab. Extracts: A Brief Review.Medicines (Basel). 2018 Aug 11;5(3):89. doi: 10.3390/medicines5030089. Medicines (Basel). 2018. PMID: 30103510 Free PMC article. Review.
-
A review of the biological effects of Myrtus communis.Physiol Rep. 2023 Jul;11(14):e15770. doi: 10.14814/phy2.15770. Physiol Rep. 2023. PMID: 37464095 Free PMC article. Review.
Cited by
-
High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders.Pharmaceuticals (Basel). 2024 Jul 23;17(8):975. doi: 10.3390/ph17080975. Pharmaceuticals (Basel). 2024. PMID: 39204080 Free PMC article. Review.
References
-
- Quinty V., Nasreddine R., Colas C., Launay A., Nehmé R., El-Khiraoui A., Piot C., Draye M., Destandau E., Da Silva D., et al. Antioxidant and anti-lipase capacities from the extracts obtained from two invasive plants: Ambrosia artemisiifolia and Solidago canadensis. Food Biosci. 2023;55:103069. doi: 10.1016/j.fbio.2023.103069. - DOI
-
- Ramos-González E.J., Bitzer-Quintero O.K., Ortiz G., Hernández-Cruz J.J., Ramírez-Jirano L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología. 2021 in press . - PubMed
-
- Bourais I., Elmarrkechy S., Taha D., Badaoui B., Mourabit Y., Salhi N., Alshahrani M.M., Al Awadh A.A., Bouyahya A., Goh K.W. Comparative Investigation of Chemical Constituents of Kernels, Leaves, Husk, and Bark of Juglans regia L., Using HPLC-DAD-ESI-MS/MS Analysis and Evaluation of Their Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. Molecules. 2022;27:8989. doi: 10.3390/molecules27248989. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

