Microbial Pathway Thermodynamics: Stoichiometric Models Unveil Anabolic and Catabolic Processes
- PMID: 38398756
- PMCID: PMC10890395
- DOI: 10.3390/life14020247
Microbial Pathway Thermodynamics: Stoichiometric Models Unveil Anabolic and Catabolic Processes
Abstract
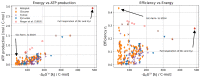
The biotechnological exploitation of microorganisms enables the use of metabolism for the production of economically valuable substances, such as drugs or food. It is, thus, unsurprising that the investigation of microbial metabolism and its regulation has been an active research field for many decades. As a result, several theories and techniques were developed that allow for the prediction of metabolic fluxes and yields as biotechnologically relevant output parameters. One important approach is to derive macrochemical equations that describe the overall metabolic conversion of an organism and basically treat microbial metabolism as a black box. The opposite approach is to include all known metabolic reactions of an organism to assemble a genome-scale metabolic model. Interestingly, both approaches are rather successful at characterizing and predicting the expected product yield. Over the years, macrochemical equations especially have been extensively characterized in terms of their thermodynamic properties. However, a common challenge when characterizing microbial metabolism by a single equation is to split this equation into two, describing the two modes of metabolism, anabolism and catabolism. Here, we present strategies to systematically identify separate equations for anabolism and catabolism. Based on metabolic models, we systematically identify all theoretically possible catabolic routes and determine their thermodynamic efficiency. We then show how anabolic routes can be derived, and we use these to approximate biomass yield. Finally, we challenge the view of metabolism as a linear energy converter, in which the free energy gradient of catabolism drives the anabolic reactions.
Keywords: elementary conversion modes; energy converter; energy metabolism; metabolic networks.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

