Between Dysbiosis, Maternal Immune Activation and Autism: Is There a Common Pathway?
- PMID: 38398873
- PMCID: PMC10891846
- DOI: 10.3390/nu16040549
Between Dysbiosis, Maternal Immune Activation and Autism: Is There a Common Pathway?
Abstract
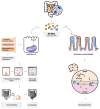
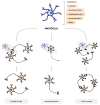
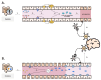
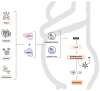
Autism spectrum disorder (ASD) is a neuropsychiatric condition characterized by impaired social interactions and repetitive stereotyped behaviors. Growing evidence highlights an important role of the gut-brain-microbiome axis in the pathogenesis of ASD. Research indicates an abnormal composition of the gut microbiome and the potential involvement of bacterial molecules in neuroinflammation and brain development disruptions. Concurrently, attention is directed towards the role of short-chain fatty acids (SCFAs) and impaired intestinal tightness. This comprehensive review emphasizes the potential impact of maternal gut microbiota changes on the development of autism in children, especially considering maternal immune activation (MIA). The following paper evaluates the impact of the birth route on the colonization of the child with bacteria in the first weeks of life. Furthermore, it explores the role of pro-inflammatory cytokines, such as IL-6 and IL-17a and mother's obesity as potentially environmental factors of ASD. The purpose of this review is to advance our understanding of ASD pathogenesis, while also searching for the positive implications of the latest therapies, such as probiotics, prebiotics or fecal microbiota transplantation, targeting the gut microbiota and reducing inflammation. This review aims to provide valuable insights that could instruct future studies and treatments for individuals affected by ASD.
Keywords: autism spectrum disorders; brain–gut axis; delivery; dysbiosis; gastrointestinal; gut microbiota; maternal immune activation (MIA); microglia; neurodevelopment; short-chain fatty acids (SCFA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
IUPHAR review: Targeted therapies of signaling pathways based on the gut microbiome in autism spectrum disorders: Mechanistic and therapeutic applications.Pharmacol Res. 2025 Jan;211:107559. doi: 10.1016/j.phrs.2024.107559. Epub 2024 Dec 28. Pharmacol Res. 2025. PMID: 39733842 Review.
-
The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder.Int J Mol Sci. 2022 Jan 25;23(3):1363. doi: 10.3390/ijms23031363. Int J Mol Sci. 2022. PMID: 35163286 Free PMC article. Review.
-
Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder.Pharmacol Res. 2020 Jul;157:104784. doi: 10.1016/j.phrs.2020.104784. Epub 2020 Apr 17. Pharmacol Res. 2020. PMID: 32305492 Clinical Trial.
-
The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder.Int J Mol Sci. 2019 Apr 29;20(9):2115. doi: 10.3390/ijms20092115. Int J Mol Sci. 2019. PMID: 31035684 Free PMC article. Review.
-
Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder.Sci Rep. 2019 Jan 22;9(1):287. doi: 10.1038/s41598-018-36430-z. Sci Rep. 2019. PMID: 30670726 Free PMC article.
Cited by
-
Integrative analysis identifies IL-6/JUN/MMP-9 pathway destroyed blood-brain-barrier in autism mice via machine learning and bioinformatic analysis.Transl Psychiatry. 2025 Jul 11;15(1):239. doi: 10.1038/s41398-025-03452-x. Transl Psychiatry. 2025. PMID: 40645949 Free PMC article.
-
Harnessing the Gut Microbiome: To What Extent Can Pre-/Probiotics Alleviate Immune Activation in Autism Spectrum Disorder?Nutrients. 2024 Jul 23;16(15):2382. doi: 10.3390/nu16152382. Nutrients. 2024. PMID: 39125263 Free PMC article. Review.
-
Intervention and research progress of gut microbiota-immune-nervous system in autism spectrum disorders among students.Front Microbiol. 2025 Mar 12;16:1535455. doi: 10.3389/fmicb.2025.1535455. eCollection 2025. Front Microbiol. 2025. PMID: 40143866 Free PMC article. Review.
-
The Gut-Brain-Microbiota Connection and Its Role in Autism Spectrum Disorders.Nutrients. 2025 Mar 25;17(7):1135. doi: 10.3390/nu17071135. Nutrients. 2025. PMID: 40218893 Free PMC article. Review.
-
Identification of Bacterial Lipopolysaccharide-Associated Genes and Molecular Subtypes in Autism Spectrum Disorder.Pharmgenomics Pers Med. 2025 Jan 17;18:1-18. doi: 10.2147/PGPM.S494126. eCollection 2025. Pharmgenomics Pers Med. 2025. PMID: 39850061 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

