Versatile Peptide-Based Nanosystems for Photodynamic Therapy
- PMID: 38399272
- PMCID: PMC10892956
- DOI: 10.3390/pharmaceutics16020218
Versatile Peptide-Based Nanosystems for Photodynamic Therapy
Abstract
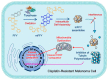
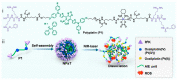
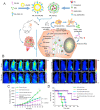
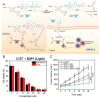
Photodynamic therapy (PDT) has become an important therapeutic strategy because it is highly controllable, effective, and does not cause drug resistance. Moreover, precise delivery of photosensitizers to tumor lesions can greatly reduce the amount of drug administered and optimize therapeutic outcomes. As alternatives to protein antibodies, peptides have been applied as useful targeting ligands for targeted biomedical imaging, drug delivery and PDT. In addition, other functionalities of peptides such as stimuli responsiveness, self-assembly, and therapeutic activity can be integrated with photosensitizers to yield versatile peptide-based nanosystems for PDT. In this article, we start with a brief introduction to PDT and peptide-based nanosystems, followed by more detailed descriptions about the structure, property, and architecture of peptides as background information. Finally, the most recent advances in peptide-based nanosystems for PDT are emphasized and summarized according to the functionalities of peptide in the system to reveal the design and development principle in different therapeutic circumstances. We hope this review could provide useful insights and valuable reference for the development of peptide-based nanosystems for PDT.
Keywords: peptide; photodynamic therapy; self-assembly; stimuli-responsive; targeting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Vermandel M., Dupont C., Lecomte F., Leroy H.A., Tuleasca C., Mordon S., Hadjipanayis C.G., Reyns N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021;152:501–514. doi: 10.1007/s11060-021-03718-6. - DOI - PubMed
-
- Usuda J., Inoue T., Tsuchida T., Ohtani K., Maehara S., Ikeda N., Ohsaki Y., Sasaki T., Oka K. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagnosis Photodyn. Ther. 2020;30:101698. doi: 10.1016/j.pdpdt.2020.101698. - DOI - PubMed
-
- Marmur E.S., Schmults C.D., Goldberg D.J. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004;30:264–271. - PubMed
-
- Frochot C., Mordon S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines. 2019;23:347–357. doi: 10.1142/S1088424619300027. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

