Repurposed Drugs That Activate Autophagy in Filarial Worms Act as Effective Macrofilaricides
- PMID: 38399310
- PMCID: PMC10891619
- DOI: 10.3390/pharmaceutics16020256
Repurposed Drugs That Activate Autophagy in Filarial Worms Act as Effective Macrofilaricides
Abstract
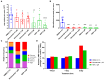
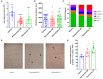
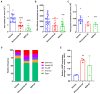
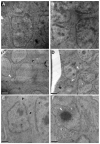
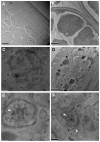
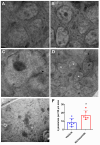
Onchocerciasis and lymphatic filariasis are two neglected tropical diseases caused by filarial nematodes that utilize insect vectors for transmission to their human hosts. Current control strategies are based on annual or biannual mass drug administration (MDA) of the drugs Ivermectin or Ivermectin plus Albendazole, respectively. These drug regimens kill the first-stage larvae of filarial worms (i.e., microfilariae) and interrupt the transmission of infections. MDA programs for these microfilaricidal drugs must be given over the lifetime of the filarial adult worms, which can reach 15 years in the case of Onchocerca volvulus. This is problematic because of suboptimal responses to ivermectin in various endemic regions and inefficient reduction of transmission even after decades of MDA. There is an urgent need for the development of novel alternative treatments to support the 2030 elimination goals of onchocerciasis and lymphatic filariasis. One successful approach has been to target Wolbachia, obligatory endosymbiotic bacteria on which filarial worms are dependent for their survival and reproduction within the human host. A 4-6-week antibiotic therapy with doxycycline, for example, resulted in the loss of Wolbachia that subsequently led to extensive apoptosis of somatic cells, germline, embryos, and microfilariae, as well as inhibition of fourth-stage larval development. However, this long-course regimen has limited use in MDA programs. As an alternative approach to the use of bacteriostatic antibiotics, in this study, we focused on autophagy-inducing compounds, which we hypothesized could disturb various pathways involved in the interdependency between Wolbachia and filarial worms. We demonstrated that several such compounds, including Niclosamide, an FDA-approved drug, Niclosamide ethanolamine (NEN), and Rottlerin, a natural product derived from Kamala trees, significantly reduced the levels of Wolbachia in vitro. Moreover, when these compounds were used in vivo to treat Brugia pahangi-infected gerbils, Niclosamide and NEN significantly decreased adult worm survival, reduced the release of microfilariae, and decreased embryonic development depending on the regimen and dose used. All three drugs given orally significantly reduced Wolbachia loads and induced an increase in levels of lysosome-associated membrane protein in worms from treated animals, suggesting that Niclosamide, NEN, and Rottlerin were effective in causing drug-induced autophagy in these filarial worms. These repurposed drugs provide a new avenue for the clearance of adult worms in filarial infections.
Keywords: Wolbachia; autophagy; filarial diseases; macrofilaricidal drugs; repurposed drugs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections.PLoS Negl Trop Dis. 2020 Dec 7;14(12):e0008930. doi: 10.1371/journal.pntd.0008930. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33284808 Free PMC article.
-
The Eagle effect in the Wolbachia-worm symbiosis.Parasit Vectors. 2021 Feb 24;14(1):118. doi: 10.1186/s13071-020-04545-w. Parasit Vectors. 2021. PMID: 33627171 Free PMC article.
-
The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target.PLoS Negl Trop Dis. 2009 Jul 14;3(7):e475. doi: 10.1371/journal.pntd.0000475. PLoS Negl Trop Dis. 2009. PMID: 19597542 Free PMC article.
-
Anti-Wolbachia therapy for onchocerciasis & lymphatic filariasis: Current perspectives.Indian J Med Res. 2019 Jun;149(6):706-714. doi: 10.4103/ijmr.IJMR_454_17. Indian J Med Res. 2019. PMID: 31496523 Free PMC article. Review.
-
Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis.Parasitology. 2014 Jan;141(1):119-27. doi: 10.1017/S0031182013001108. Epub 2013 Jul 18. Parasitology. 2014. PMID: 23866958 Free PMC article. Review.
Cited by
-
Time to consider doxycycline in the standard treatment of lymphatic filariasis? Emerging evidence on use of doxycycline as an adjunct to hygiene protocols.Trop Med Health. 2025 Mar 24;53(1):39. doi: 10.1186/s41182-025-00726-4. Trop Med Health. 2025. PMID: 40122880 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

