Y-Site Compatibility Studies of Parenteral Nutrition and Other Intravenous Medications in Neonatal and Pediatric Patients: A Review of the Literature Evidence
- PMID: 38399318
- PMCID: PMC10892144
- DOI: 10.3390/pharmaceutics16020264
Y-Site Compatibility Studies of Parenteral Nutrition and Other Intravenous Medications in Neonatal and Pediatric Patients: A Review of the Literature Evidence
Abstract
Background: Polytherapy in neonatal and pediatric patients requiring parenteral nutrition (PN) administration is a challenging task. Due to limited intravenous access, the Y-site administration of medication with PN admixtures is sometimes inevitable.
Aim: This review aims to summarize the evidence on the compatibility of the Y-site of intravenous medications and PN admixtures in neonatal and pediatric settings.
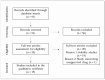
Methods: A literature review of the PubMed database was conducted. Articles published between January 1995 and November 2023 concerning the compatibility of intravenous medications in pediatric-dose PN admixtures or with intravenous lipid emulsions only were included. Studies concerning the compatibility/stability of the ingredients of PN admixtures and those concerning unapproved medications were excluded. Based on the methodology used, the quality of the research was assessed.
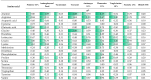
Results: A total of fifteen studies were explored. Among fifty-five different drug substances assessed in the research reviewed, 56% (31/55) were found to be compatible, 13% (7/55) were assigned as incompatible, and for 31% (17/55), the data were ambiguous. None of the studies demonstrated an "A" grade (very high quality), and the grades "B", "C", and "D" were assigned to four, six, and five studies, respectively. The compatibility data are presented in two tables, the first concerning the simultaneous administration of medications with 2-in-1 PN formulations (without lipids) and the second, with 3-in-1 formulations (with lipids) and lipid emulsions.
Conclusions: This review presents data on compatibilities between intravenously administered medications and PN mixtures intended for neonates and pediatric patients found in the PubMed database. It should be highlighted, however, that this work has some limitations. The clinical decisions on the simultaneous administration of intravenous medication with PN admixtures should be based not only on this review (including assessment of the quality of evidence) but also on manufacturer data, available electronic databases, and incompatibility data for PN admixtures dedicated to adult patients.
Keywords: compatibility; intravenous drugs; parenteral nutrition; pediatric patients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Y-Site Compatibility Studies of Ketoprofen with Parenteral Nutrition Admixtures for Central and Peripheral Administration.Pharmaceutics. 2022 Nov 23;14(12):2570. doi: 10.3390/pharmaceutics14122570. Pharmaceutics. 2022. PMID: 36559064 Free PMC article.
-
Physicochemical Stability and Sterility of Standard Parenteral Nutrition Solutions and Simulated Y-Site Admixtures for Neonates.Nutr Clin Pract. 2018 Oct;33(5):694-700. doi: 10.1002/ncp.10013. Epub 2018 Feb 21. Nutr Clin Pract. 2018. PMID: 29464781
-
Compatibility of intravenous medications with parenteral nutrition: in vitro evaluation.JPEN J Parenter Enteral Nutr. 2013 May-Jun;37(3):416-24. doi: 10.1177/0148607112464239. Epub 2012 Oct 30. JPEN J Parenter Enteral Nutr. 2013. PMID: 23112277
-
Three-in-one parenteral nutrition in neonates and pediatric patients: risks and benefits.Nutr Clin Pract. 2015 Jun;30(3):337-43. doi: 10.1177/0884533615580596. Epub 2015 Apr 9. Nutr Clin Pract. 2015. PMID: 25857309 Review.
-
Parenteral nutrition compatibility and stability: Practical considerations.Nutr Clin Pract. 2024 Oct;39(5):1150-1163. doi: 10.1002/ncp.11189. Epub 2024 Jul 12. Nutr Clin Pract. 2024. PMID: 38994914 Review.
Cited by
-
Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition.Pharmaceuticals (Basel). 2024 Jul 5;17(7):896. doi: 10.3390/ph17070896. Pharmaceuticals (Basel). 2024. PMID: 39065746 Free PMC article.
References
-
- Institute for Safe Medication Practices . [High Alert Drug] Institute for Safe Medication Practices List of High-Alert Medications in Acute Care Settings. Institute for Safe Medication Practices; Buttler Pike, PA, USA: 2018.
-
- Riskin A., Picaud J.-C., Shamir R., ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Standard versus individualized parenteral nutrition. Clin. Nutr. 2018;37:2409–2417. doi: 10.1016/j.clnu.2018.06.955. - DOI - PubMed
-
- Koletzko B., Bhutta Z.A., Cai W., Cruchet S., El Guindi M., Fuchs G.J., Goddard E.A., van Goudoever J.B., Quak S.H., Kulkarny B., et al. Compositional requirements of follow-up formula for use in infancy: Recommendations of an international expert group coordinated by the Early Nutrition Academy. Ann. Nutr. Metab. 2013;62:44–54. doi: 10.1159/000345906. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

