Relationships between Patient-Reported Outcome Measures and Clinical Measures in Naïve Neovascular Age-Related Macular Degeneration Patients Treated with Intravitreal Ranibizumab
- PMID: 38399372
- PMCID: PMC10893278
- DOI: 10.3390/ph17020157
Relationships between Patient-Reported Outcome Measures and Clinical Measures in Naïve Neovascular Age-Related Macular Degeneration Patients Treated with Intravitreal Ranibizumab
Abstract
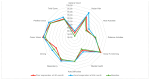
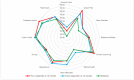
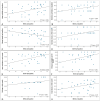
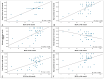
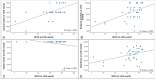
Our objective was to evaluate changes in patient-reported outcome measures using the NEI-VFQ 25 questionnaire during a treat and extend regimen in naive neovascular Age-Related Macular Degeneration patients, and its correlation with anatomical and functional data. We conducted a prospective observational study. Patients underwent a treat and extend regimen with intravitreal ranibizumab for neovascular Age-Related Macular Degeneration. Initial response was evaluated at 4th month, and subsequently in every follow-up visit. If a clinical response was achieved, the injection interval was extended in two-week increments, up to a maximum of 12 weeks. Quality of life was assessed using the NEI-VFQ 25 questionnaire at baseline, 4th months, and 12th months. Patients were categorized as good or poor responders based on Best corrected visual acuity, central foveal thickness, intraretinal fluid, or subretinal fluid. Treatment with ranibizumab led to a significant improvement in quality of life, with a mean increase in NEI-VFQ 25 score of 4.27 points in the 12th month. No significant differences in improvement were observed between good and poor responders. Quality of life scores in neovascular Age-Related Macular Degeneration patients improved with intravitreal treatment regardless of the clinical response. The early response following the loading phase could indicate better quality of life after one year of treatment, with Best corrected visual acuity being the clinical parameter with the greatest influence on quality of life.
Keywords: age-related macular degeneration; anti-VEGF; patient-reported outcome measures; quality of life; treat and extend.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Schmidt-Erfurth U., Chong V., Loewenstein A., Larsen M., Souied E., Schlingemann R., Eldem B., Monés J., Richard G., Bandello F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA) Br. J. Ophthalmol. 2014;98:1144–1167. doi: 10.1136/bjophthalmol-2014-305702. - DOI - PMC - PubMed
-
- Finger R.P., Daien V., Eldem B.M., Talks J.S., Korobelnik J.-F., Mitchell P., Sakamoto T., Wong T.Y., Pantiri K., Carrasco J. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration—A systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20:294. doi: 10.1186/s12886-020-01554-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

