Cohort Profile: The Zurich Primary HIV Infection Study
- PMID: 38399706
- PMCID: PMC10893142
- DOI: 10.3390/microorganisms12020302
Cohort Profile: The Zurich Primary HIV Infection Study
Abstract
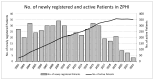
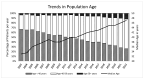
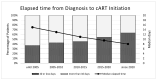
The Zurich Primary HIV Infection (ZPHI) study is a longitudinal cohort study established in 2002, aiming to study the clinical, epidemiological, and biological characteristics of primary HIV infection. The ZPHI enrolls individuals with documented primary HIV-1 infection. At the baseline and thereafter, the socio-demographic, clinical, and laboratory data are systematically collected, and regular blood sampling is performed for biobanking. By the end of December 2022, 486 people were enrolled, of which 353 were still undergoing active follow-up. Of the 486 participants, 86% had an acute infection, and 14% a recent HIV-1 infection. Men who have sex with men accounted for 74% of the study population. The median time from the estimated date of infection to diagnosis was 32 days. The median time from diagnosis to the initiation of antiretroviral therapy was 11 days, and this has consistently decreased over the last two decades. During the seroconversion phase, 447 (92%) patients reported having symptoms, of which only 73% of the patients were classified as having typical acute retroviral syndrome. The ZPHI study is a well-characterized cohort belonging to the most extensively studied primary HIV infection cohort. Its findings contribute to advancing our understanding of the early stages of HIV infection and pathogenesis, and it is paving the way to further improve HIV translational research and HIV medicine.
Keywords: HIV; acute HIV infection; acute retroviral syndrome; clinical presentation; cohort profile; primary HIV infection; recent HIV infection; sexually transmitted diseases.
Conflict of interest statement
D.L.B. reports honoraria for advisory boards, lectures and travel grants paid to himself from the companies Gilead, MSD and ViiV, which are unrelated to the submitted work. H.F.G. reports honoraria for advisory boards and DSMB from Gilead, Merck, ViiV, GSK, Johnson and Johnson, and Janssen and Novartis, which are unrelated to the submitted work. In addition, he has received unrestricted research grants from Gilead (money paid to the institution). All the other authors declare no conflicts of interest.
Figures
References
-
- Collaboration H.-C., Ray M., Logan R., Sterne J.A., Hernandez-Diaz S., Robins J.M., Sabin C., Bansi L., van Sighem A., de Wolf F., et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. - DOI - PMC - PubMed
-
- Marcus J.L., Leyden W.A., Alexeeff S.E., Anderson A.N., Hechter R.C., Hu H., Lam J.O., Towner W.J., Yuan Q., Horberg M.A., et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults with and without HIV Infection, 2000–2016. JAMA Netw. Open. 2020;3:e207954. doi: 10.1001/jamanetworkopen.2020.7954. - DOI - PMC - PubMed
-
- Takata H., Buranapraditkun S., Kessing C., Fletcher J.L., Muir R., Tardif V., Cartwright P., Vandergeeten C., Bakeman W., Nichols C.N., et al. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci. Transl. Med. 2017;9:eaag1809. doi: 10.1126/scitranslmed.aag1809. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

