Development of Stable Packaging and Producer Cell Lines for the Production of AAV Vectors
- PMID: 38399788
- PMCID: PMC10892526
- DOI: 10.3390/microorganisms12020384
Development of Stable Packaging and Producer Cell Lines for the Production of AAV Vectors
Abstract
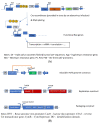
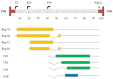
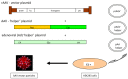
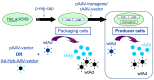
Today, recombinant adeno-associated virus (rAAV) vectors represent the vector systems which are mostly used for in vivo gene therapy for the treatment of rare and less-rare diseases. Although most of the past developments have been performed by using a transfection-based method and more than half of the authorized rAAV-based treatments are based on transfection process, the tendency is towards the use of stable inducible packaging and producer cell lines because their use is much more straightforward and leads in parallel to reduction in the overall manufacturing costs. This article presents the development of HeLa cell-based packaging/producer cell lines up to their use for large-scale rAAV vector production, the more recent development of HEK293-based packaging and producer cell lines, as well as of packaging cell lines based on the use of Sf9 cells. The production features are presented in brief (where available), including vector titer, specific productivity, and full-to-empty particle ratio.
Keywords: CAP cells; HEK293 cells; HeLa cells; Sf9 cells; adenovirus; baculovirus; herpes simplex virus; rAAV; stable inducible packaging and producer cell lines.
Conflict of interest statement
Author Otto Wilhelm Merten is employed by the company Miltenyi Biotec SAS. The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap.J Virol. 1997 Nov;71(11):8780-9. doi: 10.1128/JVI.71.11.8780-8789.1997. J Virol. 1997. PMID: 9343238 Free PMC article.
-
High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap.Gene Ther. 1999 Jun;6(6):986-93. doi: 10.1038/sj.gt.3300937. Gene Ther. 1999. PMID: 10455400
-
OneBac 2.0: Sf9 Cell Lines for Production of AAV1, AAV2, and AAV8 Vectors with Minimal Encapsidation of Foreign DNA.Hum Gene Ther Methods. 2017 Feb;28(1):15-22. doi: 10.1089/hgtb.2016.164. Hum Gene Ther Methods. 2017. PMID: 28125901 Free PMC article.
-
Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms.Biotechnol J. 2017 Mar;12(3). doi: 10.1002/biot.201600193. Epub 2017 Feb 8. Biotechnol J. 2017. PMID: 28177193 Review.
-
Adenovirus-adeno-associated virus hybrid for large-scale recombinant adeno-associated virus production.Hum Gene Ther. 2009 Sep;20(9):922-9. doi: 10.1089/hum.2009.125. Hum Gene Ther. 2009. PMID: 19618999 Review.
Cited by
-
Quantitative proteomic analysis of residual host cell protein retention across adeno-associated virus affinity chromatography.Mol Ther Methods Clin Dev. 2024 Nov 18;32(4):101383. doi: 10.1016/j.omtm.2024.101383. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39691383 Free PMC article.
-
Nuclear Fraction Proteome Analyses During rAAV Production of AAV2-Plasmid-Transfected HEK-293 Cells.Int J Mol Sci. 2025 Jun 30;26(13):6315. doi: 10.3390/ijms26136315. Int J Mol Sci. 2025. PMID: 40650119 Free PMC article.
-
Suppression of toxic transgene expression by optimized artificial miRNAs increases AAV vector yields in HEK-293 cells.Mol Ther Methods Clin Dev. 2024 Jun 10;32(3):101280. doi: 10.1016/j.omtm.2024.101280. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39015407 Free PMC article.
-
Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life.Nature. 2025 Jul 30. doi: 10.1038/s41586-025-09330-2. Online ahead of print. Nature. 2025. PMID: 40739359
-
Recombinant Adeno-Associated Virus Vectors for Gene Therapy of the Central Nervous System: Delivery Routes and Clinical Aspects.Biomedicines. 2024 Jul 9;12(7):1523. doi: 10.3390/biomedicines12071523. Biomedicines. 2024. PMID: 39062095 Free PMC article. Review.
References
-
- Maguire A.M., High K.A., Auricchio A., Wright J.F., Pierce E.A., Testa F., Mingozzi F., Bennicelli J.L., Ying G.S., Rossi S., et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. - DOI - PMC - PubMed
-
- Simonelli F., Maguire A.M., Testa F., Pierce E.A., Mingozzi F., Bennicelli J.L., Rossi S., Marshall K., Banfi S., Surace E.M., et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

