Long-Chain Bio-Based Nylon 514 Salt: Crystal Structure, Phase Transformation, and Polymerization
- PMID: 38399858
- PMCID: PMC10892662
- DOI: 10.3390/polym16040480
Long-Chain Bio-Based Nylon 514 Salt: Crystal Structure, Phase Transformation, and Polymerization
Abstract
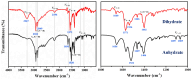
Nylon 514 is one of the new long-chain bio-based nylon materials; its raw material, 1,5-pentanediamine (PDA), is prepared by biological techniques, using biomass as the raw material. The high-performance monomer of nylon 514, 1,5-pentanediamine-tetradecanedioate (PDA-TDA) salt, was obtained through efficient crystallization methods. Here, two crystal forms of PDA-TDA, anhydrous and dihydrate, were identified and studied in this paper. From the characterization data, their crystal structures and thermal behaviors were investigated. Lattice energy was calculated to gain further insight into the relationship between thermal stability and crystal structures. The contribution of hydrogen bonds and other intermolecular interactions to the crystal structure stability have been quantified according to detailed Hirshfeld and IRI analyses. Additionally, the transformation mechanism of the anhydrate and dihydrate was established through a series of well-designed stability experiments, in which the temperature and water activity play a significant role in the structural stability of crystalline forms. Eventually, we obtained nylon 514 products with good thermal stability and low absorption using stable dihydrate powders as monomers. The properties of nylon 514 products prepared by different polymerization methods were also compared.
Keywords: 1,5-pentanediamine-tetradecanedioate; crystal structure; long-chain bio-based nylon 514 monomer; nylon polymerization; phase transformation.
Conflict of interest statement
Author Lei Zhang was employed by Nanjing Biotogether Co. Ltd. and author Huajie Lin was employed by SINOPEC Ningbo Research Institute of New Materials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures










References
-
- Lee J.A., Ahn J.H., Kim I., Li S., Lee S.Y. Synthesis, Characterization, and Application of Fully Biobased and Biodegradable Nylon-4,4 and -5,4. ACS Sustain. Chem. Eng. 2020;8:5604–5614. doi: 10.1021/acssuschemeng.0c00007. - DOI
-
- Li M. Study on melting and polymorphic behavior of poly(decamethylene terephthalamide) J. Polym. Sci. Part B Polym. Phys. 2019;57:465–472. doi: 10.1002/polb.24802. - DOI
-
- Deshmukh Y.S., Wilsens C.H.R.M., Verhoef R., Hansen M.R., Dudenko D., Graf R., Klop E.A., Rastogi S. Conformational and Structural Changes with Increasing Methylene Segment Length in Aromatic–Aliphatic Polyamides. Macromolecules. 2016;49:950–962. doi: 10.1021/acs.macromol.5b01747. - DOI
-
- Kim Y.J., Yohana K.E., Lee H.-s., Kim J. Solid-State Polymerization of Semiaromatic Copolyamides of Nylon-4,T and Nylon-4,6: Composition Ratio Effect and Thermal Properties. Ind. Eng. Chem. Res. 2012;51:15801–15810. doi: 10.1021/ie301915h. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

