Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate
- PMID: 38399872
- PMCID: PMC10893507
- DOI: 10.3390/polym16040494
Atomic Force Microscopy and Molecular Dynamic Simulation of Adsorption of Polyacrylamide with Different Chemistries onto Calcium Carbonate
Abstract
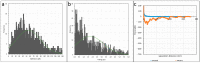
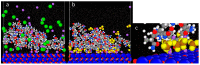
This study investigates the interaction of polyacrylamide (PAM) of different functional groups (sulfonate vs. carboxylate) and charge density (30% hydrolysed vs. 10% hydrolysed) with calcium carbonate (CaCO3) via atomic force microscopy (AFM) and partly via molecular dynamic (MD) simulations. The PAM used were F3330 (30% hydrolysed), AN125 (25% sulfonated), and AN910 (% hydrolysed). A total of 100 ppm of PAMs was prepared in 0.1% NaCl, 3% NaCl, and 4.36% NaNO3 to be employed in AFM experiments, while oligomeric models (30 repeating units) of hydrolysed polyacrylamide (HPAM), sulfonated polyacrylamide (SPAM), and neutral PAM (NPAM) were studied on a model calcite surface on MD simulations. AFM analysis indicated that F3330 has a higher average adhesion and interaction energy with CaCO3 than AN125 due to the bulky sulfonate side group of AN125 interfering with SPAM adsorption. Steric repulsion of both PAMs was similar due to their comparable molecular weights and densities of the charged group. In contrast, AN910 showed lower average adhesion and interaction energy, along with slightly longer steric repulsion with calcite than F3330, suggesting AN910 adopts more loops and tails than the slightly flatter F3330 configuration. An increase in salt concentration from 0.1% to 3% NaCl saw a reduction in adhesion and interaction energy for F3330 and AN125 due to charge screening, while AN910 saw an increase, and these values increased further at 4.36% NaNO3. MD simulations revealed that the salt ions in the system formed salt bridges between PAM and calcite, indicating that the adhesion and interaction energy observed from AFM are likely to be the net balance between PAM charged group screening and salt bridging by the salt ions present. Salt ions with larger bare radii and smaller hydrated radii were shown to form stronger salt bridges.
Keywords: adsorption; atomic force microscopy (AFM); force spectroscopy; hydrolysed polyacrylamide; molecular dynamic (MD) simulation; salt; sulfonated polyacrylamide.
Conflict of interest statement
Author Maung Maung Myo Thant is employed by the company “Petronas. (Malaysia)”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The company “Petronas. (Malaysia)” provided the funding. They were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Figures






References
-
- Wang S., Li G., Li Y., Guo J., Zhou S., Yong S., Pan B., Bai B. Adsorption of new hydrophobic polyacrylamide on the calcite surface. J. Appl. Polym. Sci. 2017;134:45314–45321. doi: 10.1002/app.45314. - DOI
-
- Mahmood A., Vissapragada B., Alghamdi A.H., Allen D., Herron M., Carnegie A., Dutta D., Olesen J.-R., Chourasiya R.D., Logan D., et al. A Snapshot of Carbonate Reservoir Evaluation. Oilfield Rev. 2000;12:20–41.
-
- Nouri A., Vaziri H., Belhaj H., Islam R. Effect of Volumetric Failure on Sand Production in Oil-Wellbores. SPE 80448; Proceedings of the SPE-Asia Pacific Oil and Gas Conference and Exhibition; Jakarta, Indonesia. 9–11 September 2003; pp. 86–93.
-
- Talaghat M.R., Esmaeilzadeh F., Mowla D. Sand production control by chemical consolidation. J. Pet. Sci. Eng. 2009;67:34–40. doi: 10.1016/j.petrol.2009.02.005. - DOI
-
- Mowar S., Zaman M., Stearns D.W., Roegiers J.C. Micro-mechanisms of pore collapse in limestone. J. Pet. Sci. Eng. 1996;15:221–235. doi: 10.1016/0920-4105(95)00065-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

