The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer-BioNTech Vaccine
- PMID: 38399963
- PMCID: PMC10893502
- DOI: 10.3390/v16020187
The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer-BioNTech Vaccine
Abstract
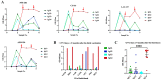
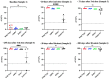
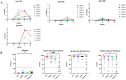
The aim of this study was to analyze the profiles of IgG subclasses in COVID-19 convalescent Puerto Rican subjects and compare these profiles with those of non-infected immunocompetent or immunocompromised subjects that received two or more doses of an mRNA vaccine. The most notable findings from this study are as follows: (1) Convalescent subjects that were not hospitalized developed high and long-lasting antibody responses. (2) Both IgG1 and IgG3 subclasses were more prevalent in the SARS-CoV-2-infected population, whereas IgG1 was more prevalent after vaccination. (3) Individuals that were infected and then later received two doses of an mRNA vaccine exhibited a more robust neutralizing capacity against Omicron than those that were never infected and received two doses of an mRNA vaccine. (4) A class switch toward the "anti-inflammatory" antibody isotype IgG4 was induced a few weeks after the third dose, which peaked abruptly and remained at high levels for a long period. Moreover, the high levels of IgG4 were concurrent with high neutralizing percentages against various VOCs including Omicron. (5) Subjects with IBD also produced IgG4 antibodies after the third dose, although these antibody levels had a limited effect on the neutralizing capacity. Knowing that the mRNA vaccines do not prevent infections, the Omicron subvariants have been shown to be less pathogenic, and IgG4 levels have been associated with immunotolerance and numerous negative effects, the recommendations for the successive administration of booster vaccinations to people should be revised.
Keywords: COVID-19; ELISA; IgG4; class switch; neutralizing antibody.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Appearance of tolerance-induction and non-inflammatory SARS-CoV-2 spike-specific IgG4 antibodies after COVID-19 booster vaccinations.Front Immunol. 2023 Dec 20;14:1309997. doi: 10.3389/fimmu.2023.1309997. eCollection 2023. Front Immunol. 2023. PMID: 38173725 Free PMC article.
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination.J Immunol Res. 2022 Aug 31;2022:4813199. doi: 10.1155/2022/4813199. eCollection 2022. J Immunol Res. 2022. PMID: 36093434 Free PMC article.
-
Persistent T cell-mediated immune responses against Omicron variants after the third COVID-19 mRNA vaccine dose.Front Immunol. 2023 Jan 23;14:1099246. doi: 10.3389/fimmu.2023.1099246. eCollection 2023. Front Immunol. 2023. PMID: 36756112 Free PMC article.
-
Neutralizing antibodies to SARS-CoV-2 Omicron variant after third mRNA vaccination in health care workers and elderly subjects.Eur J Immunol. 2022 May;52(5):816-824. doi: 10.1002/eji.202149785. Epub 2022 Mar 25. Eur J Immunol. 2022. PMID: 35312186 Free PMC article.
Cited by
-
IgG1 glycosylation highlights premature aging in Down syndrome.Aging Cell. 2024 Jul;23(7):e14167. doi: 10.1111/acel.14167. Epub 2024 Apr 15. Aging Cell. 2024. PMID: 38616780 Free PMC article.
-
A Versatile High-Throughput Single-Cell Screening Platform for Profiling Antigen-Specific Long-Lived B Cells in Blood and Bone Marrow.Adv Sci (Weinh). 2025 Jun;12(21):e2414945. doi: 10.1002/advs.202414945. Epub 2025 Apr 9. Adv Sci (Weinh). 2025. PMID: 40202243 Free PMC article.
-
COVID-19 vaccines: current and future challenges.Front Pharmacol. 2024 Nov 6;15:1434181. doi: 10.3389/fphar.2024.1434181. eCollection 2024. Front Pharmacol. 2024. PMID: 39568586 Free PMC article. Review.
-
Anti-SARS-CoV-2 IgM Antibody Levels Measured by an In-House ELISA in a Convalescent Latin Population Persist over Time and Exhibit Neutralizing Capacity to Several Variants of Concern.Diagnostics (Basel). 2024 Oct 3;14(19):2209. doi: 10.3390/diagnostics14192209. Diagnostics (Basel). 2024. PMID: 39410613 Free PMC article.
-
Patterns and functional consequences of antibody speciation in maternal-fetal transfer of coronavirus-specific humoral immunity.PLoS Pathog. 2025 Aug 6;21(8):e1013408. doi: 10.1371/journal.ppat.1013408. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40768518 Free PMC article.
References
-
- WHO WHO Coronavirus (COVID-19) Dashboard. 2022. [(accessed on 29 March 2022)]. Available online: https://covid19.who.int/
-
- Ramirez-Reveco A., Velasquez G., Aros C., Navarrete G., Villarroel-Espindola F., Navarrete M., Fica A., Plaza A., Castro N., Verdugo C., et al. Performance estimation of two in-house ELISA assays for COVID-19 surveillance through the combined detection of anti-SARS-CoV-2 IgA, IgM, and IgG immunoglobulin isotypes. PLoS ONE. 2023;18:e0270388. doi: 10.1371/journal.pone.0270388. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

