An Engineered M13 Filamentous Nanoparticle as an Antigen Carrier for a Malignant Melanoma Immunotherapeutic Strategy
- PMID: 38400008
- PMCID: PMC10893169
- DOI: 10.3390/v16020232
An Engineered M13 Filamentous Nanoparticle as an Antigen Carrier for a Malignant Melanoma Immunotherapeutic Strategy
Abstract
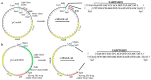
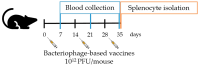
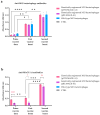
Bacteriophages, prokaryotic viruses, hold great potential in genetic engineering to open up new avenues for vaccine development. Our study aimed to establish engineered M13 bacteriophages expressing MAGE-A1 tumor peptides as a vaccine for melanoma treatment. Through in vivo experiments, we sought to assess their ability to induce robust immune responses. Using phage display technology, we engineered two M13 bacteriophages expressing MAGE-A1 peptides as fusion proteins with either pVIII or pIIII coat proteins. Mice were intraperitoneally vaccinated three times, two weeks apart, using two different engineered bacteriophages; control groups received a wild-type bacteriophage. Serum samples taken seven days after each vaccination were analyzed by ELISA assay, while splenocytes harvested seven days following the second boost were evaluated by ex vivo cytotoxicity assay. Fusion proteins were confirmed by Western blot and nano-LC-MS/MS. The application of bacteriophages was safe, with no adverse effects on mice. Engineered bacteriophages effectively triggered immune responses, leading to increased levels of anti-MAGE-A1 antibodies in proportion to the administered bacteriophage dosage. Anti-MAGE-A1 antibodies also exhibited a binding capability to B16F10 tumor cells in vitro, as opposed to control samples. Splenocytes demonstrated enhanced CTL cytotoxicity against B16F10 cells. We have demonstrated the immunogenic capabilities of engineered M13 bacteriophages, emphasizing their potential for melanoma immunotherapy.
Keywords: bacteriophage-based vaccine; filamentous bacteriophages; malignant melanoma immunotherapy; melanoma-associated antigen; nanoparticles; phage display technology.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
A virus based vaccine combined with IL12 gene therapy eradicates aggressive melanoma.Sci Rep. 2025 May 29;15(1):18786. doi: 10.1038/s41598-025-04026-z. Sci Rep. 2025. PMID: 40442309 Free PMC article.
-
The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses.J Immunol. 2008 Mar 15;180(6):3719-28. doi: 10.4049/jimmunol.180.6.3719. J Immunol. 2008. PMID: 18322177
-
Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 ScFv antibody.Colloids Surf B Biointerfaces. 2017 Nov 1;159:770-780. doi: 10.1016/j.colsurfb.2017.08.034. Epub 2017 Aug 24. Colloids Surf B Biointerfaces. 2017. PMID: 28886513
-
Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies.Pharmaceutics. 2019 Sep 1;11(9):437. doi: 10.3390/pharmaceutics11090437. Pharmaceutics. 2019. PMID: 31480551 Free PMC article. Review.
-
Unraveling the potential of M13 phages in biomedicine: Advancing drug nanodelivery and gene therapy.Environ Res. 2023 Dec 1;238(Pt 1):117132. doi: 10.1016/j.envres.2023.117132. Epub 2023 Sep 13. Environ Res. 2023. PMID: 37714365 Review.
Cited by
-
A virus based vaccine combined with IL12 gene therapy eradicates aggressive melanoma.Sci Rep. 2025 May 29;15(1):18786. doi: 10.1038/s41598-025-04026-z. Sci Rep. 2025. PMID: 40442309 Free PMC article.
-
An Immunohistochemical Study of MAGE Proteins in Hepatocellular Carcinoma.Diagnostics (Basel). 2024 Aug 5;14(15):1692. doi: 10.3390/diagnostics14151692. Diagnostics (Basel). 2024. PMID: 39125568 Free PMC article.
-
Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment.Int J Mol Sci. 2024 Sep 14;25(18):9938. doi: 10.3390/ijms25189938. Int J Mol Sci. 2024. PMID: 39337427 Free PMC article. Review.
-
Engineering M13 bacteriophage to display HER2 mimotopes on pVIII for vaccine development.Sci Rep. 2025 Jul 1;15(1):22285. doi: 10.1038/s41598-025-08032-z. Sci Rep. 2025. PMID: 40596331 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

