IE1 of Human Cytomegalovirus Inhibits Necroptotic Cell Death via Direct and Indirect Modulation of the Necrosome Complex
- PMID: 38400065
- PMCID: PMC10893529
- DOI: 10.3390/v16020290
IE1 of Human Cytomegalovirus Inhibits Necroptotic Cell Death via Direct and Indirect Modulation of the Necrosome Complex
Abstract
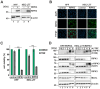
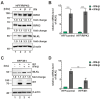
Programmed necrosis is an integral part of intrinsic immunity, serving to combat invading pathogens and restricting viral dissemination. The orchestration of necroptosis relies on a precise interplay within the necrosome complex, which consists of RIPK1, RIPK3 and MLKL. Human cytomegalovirus (HCMV) has been found to counteract the execution of necroptosis during infection. In this study, we identify the immediate-early 1 (IE1) protein as a key antagonist of necroptosis during HCMV infection. Infection data obtained in a necroptosis-sensitive cell culture system revealed a robust regulation of post-translational modifications (PTMs) of the necrosome complex as well as the importance of IE1 expression for an effective counteraction of necroptosis. Interaction analyses unveiled an association of IE1 and RIPK3, which occurs in an RHIM-domain independent manner. We propose that this interaction manipulates the PTMs of RIPK3 by promoting its ubiquitination. Furthermore, IE1 was found to exert an indirect activity by modulating the levels of MLKL via antagonizing its interferon-mediated upregulation. Overall, we claim that IE1 performs a broad modulation of innate immune signaling to impede the execution of necroptotic cell death, thereby generating a favorable environment for efficient viral replication.
Keywords: HCMV; IE1; MLKL; RIPK3; cytomegalovirus; innate immunity; interferon signaling; intrinsic immunity; necroptosis; necroptotic cell death.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Surviving death: emerging concepts of RIPK3 and MLKL ubiquitination in the regulation of necroptosis.FEBS J. 2023 Jan;290(1):37-54. doi: 10.1111/febs.16255. Epub 2021 Nov 16. FEBS J. 2023. PMID: 34710282 Review.
-
Rotavirus non-structural protein 4 usurps host cellular RIPK1-RIPK3 complex to induce MLKL-dependent necroptotic cell death.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119745. doi: 10.1016/j.bbamcr.2024.119745. Epub 2024 May 6. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38719029
-
Suppression of RIP3-dependent necroptosis by human cytomegalovirus.J Biol Chem. 2015 May 1;290(18):11635-48. doi: 10.1074/jbc.M115.646042. Epub 2015 Mar 16. J Biol Chem. 2015. PMID: 25778401 Free PMC article.
-
The regulation of necroptosis by post-translational modifications.Cell Death Differ. 2021 Mar;28(3):861-883. doi: 10.1038/s41418-020-00722-7. Epub 2021 Jan 18. Cell Death Differ. 2021. PMID: 33462412 Free PMC article. Review.
-
Reconstitution of Human Necrosome Interactions in Saccharomyces cerevisiae.Biomolecules. 2021 Jan 25;11(2):153. doi: 10.3390/biom11020153. Biomolecules. 2021. PMID: 33503908 Free PMC article.
Cited by
-
The battle between host antiviral innate immunity and immune evasion by cytomegalovirus.Cell Mol Life Sci. 2024 Aug 9;81(1):341. doi: 10.1007/s00018-024-05369-y. Cell Mol Life Sci. 2024. PMID: 39120730 Free PMC article. Review.
-
Liver cirrhosis: current status and treatment options using western or traditional Chinese medicine.Front Pharmacol. 2024 Jul 16;15:1381476. doi: 10.3389/fphar.2024.1381476. eCollection 2024. Front Pharmacol. 2024. PMID: 39081955 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

