Intradermal Fractional ChAdOx1 nCoV-19 Booster Vaccine Induces Memory T Cells: A Follow-Up Study
- PMID: 38400093
- PMCID: PMC10891531
- DOI: 10.3390/vaccines12020109
Intradermal Fractional ChAdOx1 nCoV-19 Booster Vaccine Induces Memory T Cells: A Follow-Up Study
Abstract
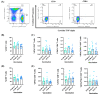
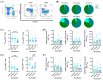
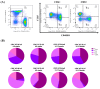
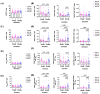
The administration of viral vector and mRNA vaccine booster effectively induces humoral and cellular immune responses. Effector T cell responses after fractional intradermal (ID) vaccination are comparable to those after intramuscular (IM) boosters. Here, we quantified T cell responses after booster vaccination. ChAdOx1 nCoV-19 vaccination induced higher numbers of S1-specific CD8+ memory T cells, consistent with the antibody responses. Effector memory T cell phenotypes elicited by mRNA vaccination showed a similar trend to those elicited by the viral vector vaccine booster. Three months post-vaccination, cytokine responses remained detectable, confirming effector T cell responses induced by both vaccines. The ID fractional dose of ChAdOx1 nCoV-19 elicited higher effector CD8+ T cell responses than IM vaccination. This study confirmed that an ID dose-reduction vaccination strategy effectively stimulates effector memory T cell responses. ID injection could be an improved approach for effective vaccination programs.
Keywords: COVID-19; SARS-CoV-2; effector T cell; intradermal; memory T cell; vaccine.
Conflict of interest statement
The authors declare no competing financial or non-financial interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

