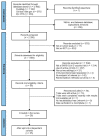
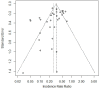
Effect of Platform Type on Clinical Efficacy of SARS-CoV-2 Vaccines in Prime Vaccination Settings: A Systematic Review and Meta-Regression of Randomized Controlled Trials
- PMID: 38400114
- PMCID: PMC10892687
- DOI: 10.3390/vaccines12020130
Effect of Platform Type on Clinical Efficacy of SARS-CoV-2 Vaccines in Prime Vaccination Settings: A Systematic Review and Meta-Regression of Randomized Controlled Trials
Abstract
This systematic review investigated the association between platform type and the clinical efficacy of SARS-CoV-2 vaccines using the meta-regression of randomized controlled trials to compare the rates of the first appearance of symptomatic COVID-19 on the platforms. The trial search was conducted using PubMed, ClinicalTrials.gov, and the EU Clinical Trials Register. The main selection criteria included: non-active control, immunocompetent individuals without previous vaccination, and a low risk of bias. The platform effect was summarized with an incidence rate ratio (IRR) and a 95% confidence interval for every platform category against the reference. IRR was obtained by random-effect meta-regression with adjustment for confounding by effect modifiers. The analysis was conducted in per-protocol (PP) and modified intention-to-treat (mITT) sets. Six vaccine types with 35 trials were included. Vector vaccines were a reference category. In the PP set, rates of symptomatic COVID-19 on mRNA and protein subunit vaccines were significantly lower than on the vector: IRR = 0.30 [0.19; 0.46], p = 0.001 and 0.63 [0.46; 0.86], p = 0.012, respectively. There was no difference for inactivated and virus-like particle vaccines compared to the vector: IRR = 0.98 [0.71; 1.36], p = 0.913 and 0.70 [0.41; 1.20], p = 0.197, respectively. The rate of cases on DNA vaccines was significantly higher than that on the vector: IRR = 2.58 [1.17; 5.68], p = 0.034. Results for the mITT set were consistent. Platform type is an effect modifier of the clinical efficacy of SARS-CoV-2 vaccines.
Keywords: COVID-19; SARS-CoV-2; coronavirus; meta-analysis; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis.BMJ. 2022 Mar 2;376:e068632. doi: 10.1136/bmj-2021-068632. BMJ. 2022. PMID: 35236664 Free PMC article.
-
Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 15;21(1):853. doi: 10.1186/s13063-020-04775-4. Trials. 2020. PMID: 33059771 Free PMC article. Clinical Trial.
-
Anti-SARS-CoV-2 spike IgG following injection of the third dose vaccine: A systematic review with meta-analysis of heterologous versus homologous vaccination.Front Public Health. 2023 Jan 12;10:960598. doi: 10.3389/fpubh.2022.960598. eCollection 2022. Front Public Health. 2023. PMID: 36711369 Free PMC article.
-
Immunogenicity, durability, and safety of an mRNA and three platform-based COVID-19 vaccines as a third dose following two doses of CoronaVac in China: A randomised, double-blinded, placebo-controlled, phase 2 trial.EClinicalMedicine. 2022 Sep 28;54:101680. doi: 10.1016/j.eclinm.2022.101680. eCollection 2022 Dec. EClinicalMedicine. 2022. PMID: 36188435 Free PMC article.
Cited by
-
A 22 month prospective assessment of neutralizing and IgG antibody levels against SARS-CoV-2 variants following homologous and heterologous BNT162b2 boosting.Sci Rep. 2025 Jul 1;15(1):21175. doi: 10.1038/s41598-025-05377-3. Sci Rep. 2025. PMID: 40594462 Free PMC article.
References
-
- Fiolet T., Kherabi Y., MacDonald C.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous