Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers
- PMID: 38400136
- PMCID: PMC10891536
- DOI: 10.3390/vaccines12020153
Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers
Abstract
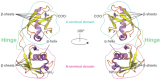
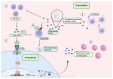
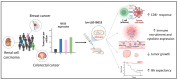
The Interferon Stimulated Gene 15 (ISG15), a unique Ubiquitin-like (Ubl) modifier exclusive to vertebrates, plays a crucial role in the immune system. Primarily induced by interferon (IFN) type I, ISG15 functions through diverse mechanisms: (i) covalent protein modification (ISGylation); (ii) non-covalent intracellular action; and (iii) exerting extracellular cytokine activity. These various roles highlight its versatility in influencing numerous cellular pathways, encompassing DNA damage response, autophagy, antiviral response, and cancer-related processes, among others. The well-established antiviral effects of ISGylation contrast with its intriguing dual role in cancer, exhibiting both suppressive and promoting effects depending on the tumour type. The multifaceted functions of ISG15 extend beyond intracellular processes to extracellular cytokine signalling, influencing immune response, chemotaxis, and anti-tumour effects. Moreover, ISG15 emerges as a promising adjuvant in vaccine development, enhancing immune responses against viral antigens and demonstrating efficacy in cancer models. As a therapeutic target in cancer treatment, ISG15 exhibits a double-edged nature, promoting or suppressing oncogenesis depending on the tumour context. This review aims to contribute to future studies exploring the role of ISG15 in immune modulation and cancer therapy, potentially paving the way for the development of novel therapeutic interventions, vaccine development, and precision medicine.
Keywords: IFN; ISG15; ISGylation; adjuvant; cytokines; immunity; inflammation; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

