Development of NP-Based Universal Vaccine for Influenza A Viruses
- PMID: 38400140
- PMCID: PMC10892571
- DOI: 10.3390/vaccines12020157
Development of NP-Based Universal Vaccine for Influenza A Viruses
Abstract
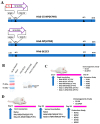
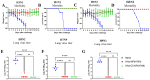
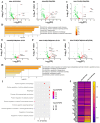
The nucleoprotein (NP) is a vital target for the heterosubtypic immunity of CD8+ cytotoxic T lymphocytes (CTLs) due to its conservation among influenza virus subtypes. To further enhance the T cell immunity of NP, autophagy-inducing peptide C5 (AIP-C5) from the CFP10 protein of Mycobacterium tuberculosis was used. Mice were immunized intranasally (i.n.) with human adenoviral vectors, HAd-C5-NP(H7N9) or HAd-NP(H7N9), expressing NP of an H7N9 influenza virus with or without the AIP-C5, respectively. Both vaccines developed similar levels of NP-specific systemic and mucosal antibody titers; however, there was a significantly higher number of NP-specific CD8 T cells secreting interferon-gamma (IFN-γ) in the HAd-C5-NP(H7N9) group than in the HAd-NP(H7N9) group. The HAd-C5-NP(H7N9) vaccine provided better protection following the challenge with A/Puerto Rico/8/1934(H1N1), A/Hong Kong/1/68(H3N2), A/chukkar/MN/14951-7/1998(H5N2), A/goose/Nebraska/17097/2011(H7N9), or A/Hong Kong/1073/1999(H9N2) influenza viruses compared to the HAd-NP(H7N9) group. The autophagy transcriptomic gene analysis of the HAd-C5-NP(H7N9) group revealed the upregulation of some genes involved in the positive regulation of the autophagy process. The results support further exploring the use of NP and AIP-C5 for developing a universal influenza vaccine for pandemic preparedness.
Keywords: adenoviral vector; autophagy; autophagy-inducing peptide; influenza vaccine; nucleoprotein; universal influenza vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Adenoviral Vector-Based Vaccine Expressing Hemagglutinin Stem Region with Autophagy-Inducing Peptide Confers Cross-Protection Against Group 1 and 2 Influenza A Viruses.Vaccines (Basel). 2025 Jan 20;13(1):95. doi: 10.3390/vaccines13010095. Vaccines (Basel). 2025. PMID: 39852874 Free PMC article.
-
A bovine adenoviral-vector-based universal influenza vaccine confers protection against influenza A and B viruses in mice and ferrets.Mol Ther Nucleic Acids. 2025 Jun 9;36(3):102594. doi: 10.1016/j.omtn.2025.102594. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40635672 Free PMC article.
-
Effect of an Adenovirus-Vectored Universal Influenza Virus Vaccine on Pulmonary Pathophysiology in a Mouse Model.J Virol. 2021 Apr 12;95(9):e02359-20. doi: 10.1128/JVI.02359-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33627390 Free PMC article.
-
Progress on adenovirus-vectored universal influenza vaccines.Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review.
-
Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines.Vaccines (Basel). 2020 Oct 1;8(4):574. doi: 10.3390/vaccines8040574. Vaccines (Basel). 2020. PMID: 33019589 Free PMC article. Review.
Cited by
-
A multi-antigen-based SARS-CoV-2 vaccine provides higher immune responses and protection against SARS-CoV-2 variants.NPJ Vaccines. 2025 Jul 19;10(1):159. doi: 10.1038/s41541-025-01198-7. NPJ Vaccines. 2025. PMID: 40683879 Free PMC article.
-
Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2.Vaccines (Basel). 2025 Apr 14;13(4):406. doi: 10.3390/vaccines13040406. Vaccines (Basel). 2025. PMID: 40333307 Free PMC article. Review.
-
Rapid Development of Modified Vaccinia Virus Ankara (MVA)-Based Vaccine Candidates Against Marburg Virus Suitable for Clinical Use in Humans.Vaccines (Basel). 2024 Nov 24;12(12):1316. doi: 10.3390/vaccines12121316. Vaccines (Basel). 2024. PMID: 39771978 Free PMC article.
-
Analysis of polyclonal and monoclonal antibody to the influenza virus nucleoprotein in different oligomeric states.bioRxiv [Preprint]. 2024 Sep 12:2024.09.12.612748. doi: 10.1101/2024.09.12.612748. bioRxiv. 2024. Update in: Virus Res. 2025 May;355:199563. doi: 10.1016/j.virusres.2025.199563. PMID: 39372734 Free PMC article. Updated. Preprint.
-
Adenoviral Vector-Based Vaccine Expressing Hemagglutinin Stem Region with Autophagy-Inducing Peptide Confers Cross-Protection Against Group 1 and 2 Influenza A Viruses.Vaccines (Basel). 2025 Jan 20;13(1):95. doi: 10.3390/vaccines13010095. Vaccines (Basel). 2025. PMID: 39852874 Free PMC article.
References
-
- WHO Influenza (Seasonal) [(accessed on 26 January 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- WHO Influenza (Avian and Other Zoonotic) [(accessed on 21 September 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-ot...
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

