The Dynamic Risk of COVID-19-Related Events in Vaccinated Healthcare Workers (HCWs) from a Tertiary Hospital in Bucharest, Romania: A Study Based on Active Surveillance Data
- PMID: 38400165
- PMCID: PMC10891893
- DOI: 10.3390/vaccines12020182
The Dynamic Risk of COVID-19-Related Events in Vaccinated Healthcare Workers (HCWs) from a Tertiary Hospital in Bucharest, Romania: A Study Based on Active Surveillance Data
Abstract
Our study describes the frequency and severity of COVID-19 in HCWs and estimates the dynamic risk of COVID-19-related events. We actively surveyed all HCWs from a tertiary infectious disease hospital from 26 February 2020 to 31 May 2023. Of 1220 HCWs, 62.9% (767) had at least one COVID-19 episode. The under 29 years (p = 0.0001) and 40-49 years (p = 0.01) age groups, nurses (p = 0.0001), and high-risk departments (p = 0.037) were characteristics significantly more frequent in HCWs with COVID-19 history. A higher percentage of boosters (53.2%; p < 0.0001) were registered in the uninfected group. The second episode of COVID-19 was significantly milder than the first. Data regarding clinical outcomes from 31 January 2021 to 31 May 2023 were analyzed in a follow-up study to determine the risk of COVID-19-related events. The Cox regression analysis revealed that HCWs with booster shots had a lower risk of COVID-19 across all events, symptomatic events, and moderate to severe events as adjusted hazard ratio (aHR) were: 0.71 (95%CI: 0.54-0.96), 0.23 (95%CI: 0.12-0.46), and 0.17 (95%CI: 0.07-0.43), respectively. Within the vaccinated subgroup, the HCWs with hybrid immunity and booster had aHR for all followed-up events of 0.42 (95%CI: 0.30-0.58), for symptomatic events of 0.52 (95%CI: 0.36-0.74), and 0.15 (95%CI: 0.03-0.66) for moderate to severe events. The risk of COVID-19 clinical events was lower for HCWs with at least one booster than those completely vaccinated.
Keywords: COVID-19; clinical outcome; healthcare workers; vaccines protection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
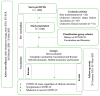
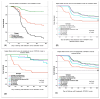
References
-
- Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. [(accessed on 9 December 2023)]. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet....
-
- Interim Recommendations for the Use of mRNA COVID-19 Vaccines. [(accessed on 9 December 2023)]. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-....
-
- Interim Recommendations for the Use of Inactivated COVID-19 Vaccines. [(accessed on 9 December 2023)]. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-....
-
- WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines: An Approach to Optimize the Global Impact of COVID-19 Vaccines, Based on Public Health Goals, Global and National Equity, and Vaccine Access and Coverage Scenarios (Version 21 January 2022)—World|ReliefWeb. [(accessed on 9 December 2023)]. Available online: https://reliefweb.int/report/world/who-sage-roadmap-prioritizing-uses-co....
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 9 December 2023)]. Available online: https://covid19.who.int.
LinkOut - more resources
Full Text Sources

