Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study
- PMID: 38400166
- PMCID: PMC10893330
- DOI: 10.3390/vaccines12020183
Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study
Abstract
Background: Long COVID has become a central public health concern. This study characterized the effectiveness of BNT162b2 BA.4/5 bivalent COVID-19 vaccine (bivalent) against long COVID symptoms.
Methods: Symptomatic US adult outpatients testing positive for SARS-CoV-2 were recruited between 2 March and 18 May 2023. Symptoms were assessed longitudinally using a CDC-based symptom questionnaire at Week 4, Month 3, and Month 6 following infection. The odds ratio (OR) of long COVID between vaccination groups was assessed by using mixed-effects logistic models, adjusting for multiple covariates.
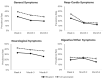
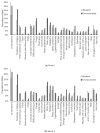
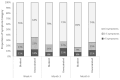
Results: At Week 4, among 505 participants, 260 (51%) were vaccinated with bivalent and 245 (49%) were unvaccinated. Mean age was 46.3 years, 70.7% were female, 25.1% had ≥1 comorbidity, 43.0% prior infection, 23.0% reported Nirmatrelvir/Ritonavir use. At Month 6, the bivalent cohort had 41% lower risk of long COVID with ≥3 symptoms (OR: 0.59, 95% CI, 0.36-0.96, p = 0.034) and 37% lower risk of ≥2 symptoms (OR: 0.63, 95% CI, 0.41-0.96, p = 0.030). The bivalent cohort reported fewer and less durable symptoms throughout the six-month follow-up, driven by neurologic and general symptoms, especially fatigue.
Conclusions: Compared with unvaccinated participants, participants vaccinated with the bivalent were associated with approximately 40% lower risk of long COVID and less symptom burden over the six-month study duration.
Keywords: BA.4/5; BNT162b2; COVID-19; COVID-19 symptoms; PASC; SARS-CoV-2; bivalent; long COVID.
Conflict of interest statement
M.D.F., A.Y., M.B.A., K.E.A., T.M.P., L.P., J.R., S.M.C.L. and J.C.C. are employees of Pfizer and may hold stock or stock options of Pfizer. X.S. and A.B. are employees of CVS Health and may hold stock of CVS Health.
Figures

Similar articles
-
Impact of Bivalent BA.4/5 BNT162b2 COVID-19 Vaccine on Acute Symptoms, Quality of Life, Work Productivity and Activity Levels among Symptomatic US Adults Testing Positive for SARS-CoV-2 at a National Retail Pharmacy.Vaccines (Basel). 2023 Oct 31;11(11):1669. doi: 10.3390/vaccines11111669. Vaccines (Basel). 2023. PMID: 38006001 Free PMC article.
-
Impact of COVID-19 and effects of booster vaccination with BNT162b2 on six-month long COVID symptoms, quality of life, work productivity and activity impairment during Omicron.J Patient Rep Outcomes. 2023 Jul 24;7(1):77. doi: 10.1186/s41687-023-00616-5. J Patient Rep Outcomes. 2023. PMID: 37486567 Free PMC article.
-
Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: a test-negative case-control study.Lancet Respir Med. 2023 Dec;11(12):1089-1100. doi: 10.1016/S2213-2600(23)00306-5. Epub 2023 Oct 25. Lancet Respir Med. 2023. PMID: 37898148
-
Impact of COVID-19 and effects of BNT162b2 on patient-reported outcomes: quality of life, symptoms, and work productivity among US adult outpatients.J Patient Rep Outcomes. 2022 Dec 5;6(1):123. doi: 10.1186/s41687-022-00528-w. J Patient Rep Outcomes. 2022. PMID: 36469198 Free PMC article.
-
Real-world evidence on the efficacy of bivalent booster doses of SARS-CoV-2 vaccine in respect of monovalent boosters or primary cycle of vaccination: a narrative review.Epidemiol Prev. 2023 Nov-Dec;47(6):331-343. doi: 10.19191/EP23.6.A626.081. Epidemiol Prev. 2023. PMID: 38314543 Review. English.
Cited by
-
Prospective cohort study of fatigue before and after SARS-CoV-2 infection in the Netherlands.Nat Commun. 2025 Mar 4;16(1):1923. doi: 10.1038/s41467-025-56994-5. Nat Commun. 2025. PMID: 40038286 Free PMC article.
-
Factors affecting the impact of COVID-19 vaccination on post COVID-19 conditions among adults: A systematic literature review.Hum Vaccin Immunother. 2025 Dec;21(1):2474772. doi: 10.1080/21645515.2025.2474772. Epub 2025 Mar 13. Hum Vaccin Immunother. 2025. PMID: 40079963 Free PMC article.
-
Predictors of Long COVID Among Symptomatic US Adults Testing Positive for SARS-CoV-2 at a National Retail Pharmacy.Healthcare (Basel). 2024 Nov 21;12(23):2321. doi: 10.3390/healthcare12232321. Healthcare (Basel). 2024. PMID: 39684943 Free PMC article.
-
Multidisciplinary collaborative guidance on the assessment and treatment of patients with Long COVID: A compendium statement.PM R. 2025 Jun;17(6):684-708. doi: 10.1002/pmrj.13397. Epub 2025 Apr 22. PM R. 2025. PMID: 40261198
References
-
- Centers for Disease Control and Prevention Long COVID or Post-COVID Conditions. [(accessed on 11 December 2023)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
-
- Gottlieb M., Wang R., Yu H., Spatz E.S., Montoy J.C., Rodriguez R., Chang A.M., Elmore J.G., Hannikainen P.A., Hill M. Severe Fatigue and Persistent Symptoms at Three Months Following SARS-CoV-2 Infections During the Pre-Delta, Delta, and Omicron Time Periods: A Multicenter Prospective Cohort Study. Clin. Infect. Dis. 2023;76:1930–1941. doi: 10.1093/cid/ciad045. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

