Polymeric Nanoparticles as Oral and Intranasal Peptide Vaccine Delivery Systems: The Role of Shape and Conjugation
- PMID: 38400181
- PMCID: PMC10893271
- DOI: 10.3390/vaccines12020198
Polymeric Nanoparticles as Oral and Intranasal Peptide Vaccine Delivery Systems: The Role of Shape and Conjugation
Abstract
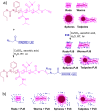
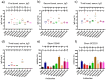
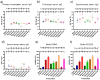
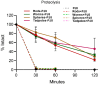
Mucosal vaccines are highly attractive due to high patient compliance and their suitability for mass immunizations. However, all currently licensed mucosal vaccines are composed of attenuated/inactive whole microbes, which are associated with a variety of safety concerns. In contrast, modern subunit vaccines use minimal pathogenic components (antigens) that are safe but typically poorly immunogenic when delivered via mucosal administration. In this study, we demonstrated the utility of various functional polymer-based nanostructures as vaccine carriers. A Group A Streptococcus (GAS)-derived peptide antigen (PJ8) was selected in light of the recent global spread of invasive GAS infection. The vaccine candidates were prepared by either conjugation or physical mixing of PJ8 with rod-, sphere-, worm-, and tadpole-shaped polymeric nanoparticles. The roles of nanoparticle shape and antigen conjugation in vaccine immunogenicity were demonstrated through the comparison of three distinct immunization pathways (subcutaneous, intranasal, and oral). No additional adjuvant or carrier was required to induce bactericidal immune responses even upon oral vaccine administration.
Keywords: Group A Streptococcus; conjugates; intranasal delivery; mucosal immunology; nanoparticles; oral delivery; peptides; physical mixture; vaccines.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus.Acta Biomater. 2018 Oct 15;80:278-287. doi: 10.1016/j.actbio.2018.09.037. Epub 2018 Sep 25. Acta Biomater. 2018. PMID: 30266637
-
[Development of polymeric nanoparticles-based vaccine].Nihon Rinsho. 2006 Feb;64(2):279-85. Nihon Rinsho. 2006. PMID: 16454182 Review. Japanese.
-
Activity Relationship of Poly(ethylenimine)-Based Liposomes as Group A Streptococcus Vaccine Delivery Systems.ACS Infect Dis. 2023 Aug 11;9(8):1570-1581. doi: 10.1021/acsinfecdis.3c00159. Epub 2023 Jul 24. ACS Infect Dis. 2023. PMID: 37489053
-
Preparation and preclinical evaluation of experimental group B streptococcus type III polysaccharide-cholera toxin B subunit conjugate vaccine for intranasal immunization.Vaccine. 2000 Nov 22;19(7-8):850-61. doi: 10.1016/s0264-410x(00)00226-7. Vaccine. 2000. PMID: 11115709
-
The influence of component structural arrangement on peptide vaccine immunogenicity.Biotechnol Adv. 2022 Nov;60:108029. doi: 10.1016/j.biotechadv.2022.108029. Epub 2022 Aug 24. Biotechnol Adv. 2022. PMID: 36028180 Review.
Cited by
-
Intranasal delivery of metformin using metal-organic framework (MOF)-74-Mg nanocarriers.Adv Compos Hybrid Mater. 2025;8(1):131. doi: 10.1007/s42114-025-01227-y. Epub 2025 Jan 18. Adv Compos Hybrid Mater. 2025. PMID: 39834534 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

