Analysis of VOCs in Liquids through Vaporization in a Tubular Oven Monitored by Chemical Ionization Mass Spectrometry
- PMID: 38400206
- PMCID: PMC10891908
- DOI: 10.3390/s24041048
Analysis of VOCs in Liquids through Vaporization in a Tubular Oven Monitored by Chemical Ionization Mass Spectrometry
Abstract
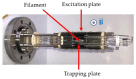
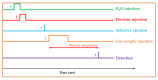
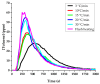
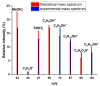
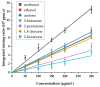
The analysis of chemical compounds present at trace levels in liquids is important not only for environmental measurements but also, for example, in the health sector. The reference technique for the analysis of Volatile Organic Compounds (VOCs) in liquids is GC, which is difficult to use with an aqueous matrix. In this work, we present an alternative technique to GC to analyze VOCs in water. A tubular oven is used to completely vaporize the liquid sample deposited on a gauze. The oven is heated in the presence of a dinitrogen flow, and the gas is analyzed at the exit of the oven by a chemical ionization mass spectrometer developed in our laboratory. It is a low magnetic field Fourier Transform Ion Cyclotron Resonance (FT-ICR) optimized for real-time analysis. The Proton Transfer Reaction (PTR) used during the Chemical Ionization event results in the selective ionization of the VOCs present in the gas phase. The optimization of the desorption conditions is described for the main operating parameters: temperature ramp, liquid quantity, and nitrogen flow. Their influence is studied using a 100 ppmv aqueous toluene solution. The analytical method is then tested on a mixture of seven VOCs.
Keywords: Fourier Transform Ion Cyclotron Resonance; mass spectrometry; proton transfer reaction; real-time analysis; tubular oven; volatile organic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Compact FTICR Mass Spectrometry for Real Time Monitoring of Volatile Organic Compounds.Sensors (Basel). 2018 May 3;18(5):1415. doi: 10.3390/s18051415. Sensors (Basel). 2018. PMID: 29751541 Free PMC article.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Proton transfer reaction-mass spectrometry applications in medical research.J Breath Res. 2009 Jun;3(2):020201. doi: 10.1088/1752-7163/3/2/020201. Epub 2009 Jun 9. J Breath Res. 2009. PMID: 21383455
-
Integration of a micropreconcentrator with solid-phase microextraction for analysis of trace volatile organic compounds by gas chromatography-mass spectrometry.J Chromatogr A. 2022 Jun 21;1673:463083. doi: 10.1016/j.chroma.2022.463083. Epub 2022 Apr 22. J Chromatogr A. 2022. PMID: 35508097 Free PMC article.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
References
-
- Kuráň P., Soják L. Environmental Analysis of Volatile Organic Compounds in Water and Sediment by Gas Chromatography. J. Chromatogr. A. 1996;733:119–141. doi: 10.1016/0021-9673(95)01121-8. - DOI
-
- Huybrechts T., Dewulf J., Moerman O., Van Langenhove H. Evaluation of Purge-and-Trap-High-Resolution Gas Chromatography-Mass Spectrometry for the Determination of 27 Volatile Organic Compounds in Marine Water at the Ng l−1 Concentration Level. J. Chromatogr. A. 2000;893:367–382. doi: 10.1016/S0021-9673(00)00771-8. - DOI - PubMed
-
- Dewulf J., Van Langenhove H., Wittmann G. Analysis of Volatile Organic Compounds Using Gas Chromatography. TrAC Trends Anal. Chem. 2002;21:637–646. doi: 10.1016/S0165-9936(02)00804-X. - DOI
-
- Cavalcante R.M., de Andrade M.V.F., Marins R.V., Oliveira L.D.M. Development of a Headspace-Gas Chromatography (HS-GC-PID-FID) Method for the Determination of VOCs in Environmental Aqueous Matrices: Optimization, Verification and Elimination of Matrix Effect and VOC Distribution on the Fortaleza Coast, Brazil. Microchem. J. 2010;96:337–343. doi: 10.1016/j.microc.2010.05.014. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

