Accessibility to ERCP-performing hospitals among patients with pancreatic cancer living in SEER regions
- PMID: 38400670
- PMCID: PMC10891451
- DOI: 10.1002/cam4.7020
Accessibility to ERCP-performing hospitals among patients with pancreatic cancer living in SEER regions
Abstract
Background and aims: The two most common interventions used to treat painless jaundice from pancreatic cancer are endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic biliary drainage (PTBD). Our study aimed to characterize the geographic distribution of ERCP-performing hospitals among patients with pancreatic cancer in the United States and the association between geographic accessibility to ERCP-performing hospitals and biliary interventions patients receive.
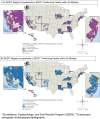
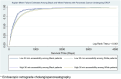
Methods: This is a retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database for pancreatic cancer from 2005 to 2013. Multilevel models were used to examine the association between accessibility to ERCP hospitals within a 30- and 45-min drive from the patient's residential ZIP Code and the receipt of ERCP treatment. A two-step floating catchment area model was used to calculate the measure of accessibility based on the distribution across SEER regions.
Results: 7464 and 782 patients underwent ERCP and PTBD, respectively, over the study period. There were 808 hospitals in which 8246 patients diagnosed with pancreatic cancer in SEER regions from 2005 to 2013 received a procedure. Patients with high accessibility within both 30- and 45-min drive to an ERCP-performing hospital were more likely to receive an ERCP (30-min adjusted odds ratio [aOR]: 1.53, 95% confidence interval [CI]: 1.17-2.01; 45-min aOR: 1.31, 95% CI: 1.01-1.70). Furthermore, in the adjusted model, Black patients (vs. White) and patients with stage IV disease were less likely to receive ERCP than PTBD.
Conclusions: Patients with pancreatic cancer and high accessibility to an ERCP-performing hospital were more likely to receive ERCP. Disparities in the receipt of ERCP persisted for Black patients regardless of their access to ERCP-performing hospitals.
Keywords: ERCP; accessibility; disparities; pancreatic cancer.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Anna Tavakkoli, Alaina Beauchamp, Tanushree Prasad, Hong Zhu, Nisa Kubiliun, Richard Kwon, and Amy Hughes disclose no conflicts of interest. Unrelated to this work, Sandi Pruitt serves as a consultant for Pfizer and Gilead, and Amit Singal has served as a consultant or on advisory boards for Genentech, AstraZeneca, Eisai, Bayer, Exelixis, Boston Scientific, Fujifilm Medical Sciences, Exact Sciences, Roche, Glycotest, Freenome, and GRAIL.
Figures
Similar articles
-
Regional and racial variations in the utilization of endoscopic retrograde cholangiopancreatography among pancreatic cancer patients in the United States.Cancer Med. 2019 Jul;8(7):3420-3427. doi: 10.1002/cam4.2225. Epub 2019 May 14. Cancer Med. 2019. PMID: 31087545 Free PMC article.
-
Survival analysis among unresectable pancreatic adenocarcinoma patients undergoing endoscopic or percutaneous interventions.Gastrointest Endosc. 2021 Jan;93(1):154-162.e5. doi: 10.1016/j.gie.2020.05.061. Epub 2020 Jun 9. Gastrointest Endosc. 2021. PMID: 32531402 Free PMC article.
-
Age, socioeconomic features, and clinical factors predict receipt of endoscopic retrograde cholangiopancreatography in pancreatic cancer.World J Gastrointest Endosc. 2019 Feb 16;11(2):133-144. doi: 10.4253/wjge.v11.i2.133. World J Gastrointest Endosc. 2019. PMID: 30788032 Free PMC article.
-
Innovations and techniques for balloon-enteroscope-assisted endoscopic retrograde cholangiopancreatography in patients with altered gastrointestinal anatomy.World J Gastroenterol. 2015 Jun 7;21(21):6460-9. doi: 10.3748/wjg.v21.i21.6460. World J Gastroenterol. 2015. PMID: 26074685 Free PMC article. Review.
-
Comparison of Efficacy and Safety between Endoscopic Retrograde Cholangiopancreatography and Percutaneous Transhepatic Cholangial Drainage for the Treatment of Malignant Obstructive Jaundice: A Systematic Review and Meta-Analysis.Digestion. 2023;104(2):85-96. doi: 10.1159/000528020. Epub 2023 Jan 6. Digestion. 2023. PMID: 36617409 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7‐33. - PubMed
-
- American Cancer Society . Cancer Facts & Figures 2023. 2023. Accessed January 25, 2023. https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐...
-
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395‐2406. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

