Phase I study of sapanisertib (CB-228/TAK-228/MLN0128) in combination with ziv-aflibercept in patients with advanced solid tumors
- PMID: 38400671
- PMCID: PMC10891443
- DOI: 10.1002/cam4.6877
Phase I study of sapanisertib (CB-228/TAK-228/MLN0128) in combination with ziv-aflibercept in patients with advanced solid tumors
Abstract
Background: Sapanisertib is a potent ATP-competitive, dual inhibitor of mTORC1/2. Ziv-aflibercept is a recombinant fusion protein comprising human VEGF receptor extracellular domains fused to human immunoglobulin G1. HIF-1α inhibition in combination with anti-angiogenic therapy is a promising anti-tumor strategy. This Phase 1 dose-escalation/expansion study assessed safety/ tolerability of sapanisertib in combination with ziv-aflibercept in advanced solid tumors.
Methods: Fifty-five patients with heavily pre-treated advanced metastatic solid tumors resistant or refractory to standard treatment received treatment on a range of dose levels.
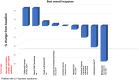
Results: Fifty-five patients were enrolled and treated across a range of dose levels. Forty were female (73%), median age was 62 (range: 21-79), and ECOG PS was 0 (9, 16%) or 1 (46, 84%). Most common tumor types included ovarian (8), colorectal (8), sarcoma (8), breast (3), cervical (4), and endometrial (4). Median number of prior lines of therapy was 4 (range 2-11). Sapanisertib 4 mg orally 3 days on and 4 days off plus 3 mg/kg ziv-aflibercept IV every 2 weeks on a 28-day cycle was defined as the maximum tolerated dose. Most frequent treatment-related grade ≥2 adverse events included hypertension, fatigue, anorexia, hypertriglyceridemia, diarrhea, nausea, mucositis, and serum lipase increase. There were no grade 5 events. In patients with evaluable disease (n = 50), 37 patients (74%) achieved stable disease (SD) as best response, two patients (4%) achieved a confirmed partial response (PR); disease control rate (DCR) (CR + SD + PR) was 78%.
Conclusion: The combination of sapanisertib and ziv-aflibercept was generally tolerable and demonstrated anti-tumor activity in heavily pre-treated patients with advanced malignancies.
Trial registration: ClinicalTrials.gov NCT02159989resesese.
Keywords: VEGF; advanced/refractory cancer; mTOR; mTORC1/2; sapanisertib; targeted therapy.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
NC, BS, SF, FO, BY, FS, and JW declare no conflicts of interest. JR reports non‐financial support and reasonable reimbursement for travel from European Society for Medical Oncology; receiving consulting and travel fees from Peptomyc, Kelun Pharmaceuticals/Klus Pharma, Ellipses Pharma, Molecular Partners, IONCTURA (including serving on the scientific advisory board); Consulting fees from Vall d'Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, Boxer Capital, LLC, Tang Advisors, LLC receiving research funding from Blueprint Medicines, Black Diamond Therapeutics, Merck Sharp & Dohme, Hummingbird, Yingli and Vall d'Hebron Institute of Oncology/Cancer Core Europe; and serving as investigator in clinical trials with Novartis, Spectrum Pharmaceuticals, Symphogen, BioAlta, Pfizer, GenMab, CytomX, Kelun‐Biotech, Takeda‐Millenium, GalxoSmithKline, Taiho, Roche Pharmaceuticals, Hummingbird, Yingli, Bycicle Therapeutics, Merus, Curis, Bayer, AadiBioscience, Nuvation, ForeBio, BioMed Valley Discoveries, Loxo Oncology, Hutchinson MediPharma, Cellestia, Deciphera, Ideaya, Amgen, Tango Therapeutics, Mirati Linnaeus Therapeutics, and Cancer Core Europe. DK reports NIH Grants (Clinical Translational Science Award 2019–2024; NCI Core Grant, Clinical Translational 2002–2023); Consulting, Black Beret Life Sciences; Advisory Board, Affigen, Phosplatin. FMB declares the following conflicts. Consulting: AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman‐La Roche Ltd., GT Apeiron, Genentech Inc., Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks; Advisory Committee: Black Diamond, Biovica, Eisai, FogPharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, Zentalis; Sponsored Research (to MD Anderson Cancer Center): Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., Taiho Pharmaceutical Co.; Honoraria: Chugai Biopharmaceuticals. Other (Travel Related) European Organization for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO). VS receives research funding for clinical trials from Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa‐sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint medicines, Loxo oncology, Takeda and Roche/ Genentech, National Comprehensive Cancer Network, NCI‐CTEP and UT MD Anderson Cancer Center. Travel: Novartis, Pharmamar, ASCO, ESMO. SPP receives Clinical Trial Research Support/Grant Funding through the institution from the following sources: AbbVie, Inc.; ABM Therapeutics, Inc.; Acepodia, Inc; Alkermes; Aminex Therapeutics; Amphivena Therapeutics, Inc.; BioMarin Pharmaceutical, Inc; Boehringer Ingelheim; Bristol Myers Squib; Cerulean Pharma, Inc.; Chugai Pharmaceutical Co., Ltd; Curis, Inc.; Cyclacel Pharmaceuticals; Daiichi Sankyo; Eli Lilly; ENB Therapeutics; Epigenetix Inc.; Five Prime Therapeutics; F‐Star Beta Limited; F‐Star Therapeutics; Gene Quantum; Genmab A/S; Gilead Sciences, Inc.; GlaxoSmithKline; Helix BioPharma Corp.; Hengrui Pharmaceuticals, Co., Ltd.; HiberCell, Inc.; Immorna Biotherapeutics, Inc.; Immunomedics, Inc.; Incyte Corp.; Jacobio Pharmaceuticals Co., Ltd.; Jiangsu Simcere Pharmaceutical Co., Ltd.; Lytix Biopharma AS; Medimmune, LLC.; Medivation, Inc.; Merck Sharp and Dohme Corp.; Nectin Therapeutics, Ltd.; Novartis Pharmaceuticals; Pieris Pharmaceuticals, Inc.; Pfizer; Phanes Therapeutics; Principia Biopharma, Inc.; Puma Biotechnology, Inc.; Purinomia Biotech, Inc.; Rapt Therapeutics, Inc.; Replimune; Seattle Genetics; Silverback Therapeutics; Synlogic Therapeutics; Taiho Oncology; Tesaro, Inc.; TransThera Bio; ZielBio, Inc.; NCI/NIH; P30CA016672 – Core Grant (CCSG Shared Resources); consultancy: CRC Oncology. AN declares the following, Research funding from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor/Millendo, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Eli Lilly, Kymab, PsiOxus, Arcus Biosciences, NeoImmuneTech, Immune‐Onc Therapeutics, Surface Oncology, Monopteros Therapeutics, BioNTech SE, Seven & Eight Biopharma, and SOTIO Biotech AG; Advisory board/Consulting fees from Deka Biosciences, NGM Bio, PsiOxus Therapeutics, Immune‐Onc Therapeutics, STCube Pharmaceuticals, OncoSec KEYNOTE‐695, Genome & Company, CytomX Therapeutics, Nouscom, Merck Sharp & Dohme Corp, OncoNano, Servier, Lynx Health, AbbVie, PsiOxus; Travel and accommodation expense from ARMO BioSciences, NeoImmuneTech, NGM Biopharmaceuticals; Honoraria for speaking engagements from AKH Inc, The Lynx Group, Society for Immunotherapy of Cancer (SITC), Korean Society of Medical Oncology (KSMO), Scripps Cancer Care Symposium, ASCO Direct Oncology Highlights, European Society for Medical Oncology (ESMO), CME Outfitters.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

