An international multicenter study comparing COVID-19 omicron outcomes in patients with hematological malignancies treated with obinutuzumab versus rituximab
- PMID: 38400683
- PMCID: PMC10891459
- DOI: 10.1002/cam4.6997
An international multicenter study comparing COVID-19 omicron outcomes in patients with hematological malignancies treated with obinutuzumab versus rituximab
Abstract
Objectives: Hematological malignancy (HM) patients treated with anti-CD20 monoclonal antibodies are at higher risk for severe COVID-19. A previous single-center study showed worse outcomes in patients treated with obinutuzumab compared to rituximab. We examined this hypothesis in a large international multicenter cohort.
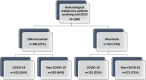
Methods: We included HM patients from 15 centers, from five countries treated with anti-CD20, comparing those treated with obinutuzumab (O-G) to rituximab (R-G) between December 2021 and June 2022, when Omicron lineage was dominant.
Results: We collected data on 1048 patients. Within the R-G (n = 762, 73%), 191 (25%) contracted COVID-19 compared to 103 (36%) in the O-G. COVID-19 patients in the O-G were younger (61 ± 11.7 vs. 64 ± 14.5, p = 0.039), had more indolent HM diagnosis (aggressive lymphoma: 3.9% vs. 67.0%, p < 0.001), and most were on maintenance therapy at COVID-19 diagnosis (63.0% vs. 16.8%, p < 0.001). Severe-critical COVID-19 occurred in 31.1% of patients in the O-G and 22.5% in the R-G. In multivariable analysis, O-G had a 2.08-fold increased risk for severe-critical COVID-19 compared to R-G (95% CI 1.13-3.84), adjusted for Charlson comorbidity index, sex, and tixagevimab/cilgavimab (T-C) prophylaxis. Further analysis comparing O-G to R-G demonstrated increased hospitalizations (51.5% vs. 35.6% p = 0.008), ICU admissions (12.6% vs. 5.8%, p = 0.042), but the nonsignificant difference in COVID-19-related mortality (n = 10, 9.7% vs. n = 12, 6.3%, p = 0.293).
Conclusions: Despite younger age and a more indolent HM diagnosis, patients receiving obinutuzumab had more severe COVID-19 outcomes than those receiving rituximab. Our findings underscore the need to evaluate the risk-benefit balance when considering obinutuzumab therapy for HM patients during respiratory viral outbreaks.
Keywords: COVID-19; anti-CD20 monoclonal antibodies; hematological malignancies; obinutuzumab; rituximab.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest with respect to this study. This study did not receive funding support.
References
-
- Calderón‐Parra J, Múñez‐Rubio E, Fernández‐Cruz A, et al. Incidence, clinical presentation, relapses and outcome of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in patients treated with anti‐CD20 monoclonal antibodies. Clin Infect Dis. 2022;74(10):1786‐1794. doi:10.1093/cid/ciab700 - DOI - PubMed
-
- WHO . Coronavirus disease situation report. World Health Organization; 2020;19(June):1.
-
- Mathieu E, Ritchie H, Rodés‐Guirao L, et al. Coronavirus Pandemic (COVID‐19). 2020. Accessed January 8, 2021. https://ourworldindata.org/coronavirus
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous