Timing of Biomarker Changes in Sporadic Alzheimer's Disease in Estimated Years from Symptom Onset
- PMID: 38400792
- PMCID: PMC11060905
- DOI: 10.1002/ana.26891
Timing of Biomarker Changes in Sporadic Alzheimer's Disease in Estimated Years from Symptom Onset
Abstract
Objective: A clock relating amyloid positron emission tomography (PET) to time was used to estimate the timing of biomarker changes in sporadic Alzheimer disease (AD).
Methods: Research participants were included who underwent cerebrospinal fluid (CSF) collection within 2 years of amyloid PET. The ages at amyloid onset and AD symptom onset were estimated for each individual. The timing of change for plasma, CSF, imaging, and cognitive measures was calculated by comparing restricted cubic splines of cross-sectional data from the amyloid PET positive and negative groups.
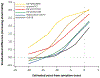
Results: The amyloid PET positive sub-cohort (n = 118) had an average age of 70.4 ± 7.4 years (mean ± standard deviation) and 16% were cognitively impaired. The amyloid PET negative sub-cohort (n = 277) included individuals with low levels of amyloid plaque burden at all scans who were cognitively unimpaired at the time of the scans. Biomarker changes were detected 15-19 years before estimated symptom onset for CSF Aβ42/Aβ40, plasma Aβ42/Aβ40, CSF pT217/T217, and amyloid PET; 12-14 years before estimated symptom onset for plasma pT217/T217, CSF neurogranin, CSF SNAP-25, CSF sTREM2, plasma GFAP, and plasma NfL; and 7-9 years before estimated symptom onset for CSF pT205/T205, CSF YKL-40, hippocampal volumes, and cognitive measures.
Interpretation: The use of an amyloid clock enabled visualization and analysis of biomarker changes as a function of estimated years from symptom onset in sporadic AD. This study demonstrates that estimated years from symptom onset based on an amyloid clock can be used as a continuous staging measure for sporadic AD and aligns with findings in autosomal dominant AD. ANN NEUROL 2024;95:951-965.
© 2024 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
YL, DY, RDH, SD, BAG, AJA, RLH, EMH, KV, MH, LJ, EM, and JCM report no disclosures relevant to the manuscript.
NRB and RJB are co-inventors on a US patent application “Methods to detect novel tau species in CSF and use thereof to track tau neuropathology in Alzheimer’s disease and other tauopathies,” and “CSF phosphorylated tau and Amyloid beta profiles as biomarkers of tauopathies.” NRB and RJB are co-inventors on a non-provisional patent application “Methods of Diagnosing and Treating Based on Site-Specific Tau Phosphorylation.”
KK, SE, and MM are employee of C2N Diagnostics.
DMH is as an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies, U.S. patent no. 9,834,596. DMH cofounded, has equity, and is on the scientific advisory board of C2N Diagnostics. DMH. is on the scientific advisory boards of Denali, Genentech, and Cajal Neuroscience and consults for Asteroid.
TLSB has investigator-initiated research funding from the NIH, the Alzheimer’s Association, the Barnes-Jewish Hospital Foundation and Siemens. She participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly, Biogen, Eisai, Jaansen, and Roche. She serves as a consultant to Biogen, Lilly, Eisai, and Siemens.
RJB co-founded C2N Diagnostics. Washington University and RJB have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics, blood plasma assay, and methods of diagnosing AD with phosphorylation changes) licensed by Washington University to C2N Diagnostics. RJB receives income from C2N Diagnostics for serving on the scientific advisory board. RJB has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb, and Novartis.
CX consulted for DIADEM and has used funding from NIH to hire C2N Diagnostics as a vendor in another independent NIH-funded project. He received no funding from C2N Diagnostics.
SES has analyzed data provided by C2N Diagnostics to Washington University. She has served on a Scientific Advisory Board for Eisai.
Figures
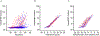




References
-
- McDade EM. Alzheimer Disease. Continuum (Minneap Minn). 2022. Jun 1;28(3):648–75. - PubMed
-
- Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. The Lancet Neurology. 2012. Aug;11(8):669–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R44 AG059489/AG/NIA NIH HHS/United States
- P01AG003991/AG/NIA NIH HHS/United States
- R01 AG070941/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01AG070941/AG/NIA NIH HHS/United States
- K23 AG053426/AG/NIA NIH HHS/United States
- P30AG066444/AG/NIA NIH HHS/United States
- RF1R01AG053550/AG/NIA NIH HHS/United States
- R01AG067505/AG/NIA NIH HHS/United States
- R44AG059489/GF/NIH HHS/United States
- P01AG026276/AG/NIA NIH HHS/United States
- K23AG053426/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG067505/AG/NIA NIH HHS/United States
- RF1 AG053550/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

