Ciliate diversity in rodrigo de freitas lagoon (Rio de Janeiro, Brazil) from an integrative standpoint
- PMID: 38401009
- PMCID: PMC11153468
- DOI: 10.1007/s42770-024-01291-4
Ciliate diversity in rodrigo de freitas lagoon (Rio de Janeiro, Brazil) from an integrative standpoint
Abstract
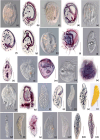
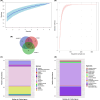
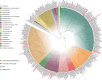
The Rodrigo de Freitas Lagoon is a highly eutrophic lacustrine system and has one of the longest histories of exploration and anthropic alteration in Brazil. Despite its relevance, limited studies explored the diversity of micro-eukaryotes in the lagoon. Ciliates (Alveolata, Ciliophora) are overlooked in environmental microbiology, especially in tropical and subtropical ecosystems, resulting in limited knowledge about their diversity and functional relevance in South American habitats, particularly in coastal lagoons. To fill this gap, here we investigated the diversity of ciliates in a brackish coastal lagoon in an urban area of Rio de Janeiro, Brazil, applying and comparing the performance of morphological and metabarcoding approaches. The metabarcoding analysis, based on high-throughput sequencing of the hipervariable region V4 of the 18S rRNA genes detected 37 molecular operational taxonomic units (MOTUs) assigned to Ciliophora, representing only about a half (56.9%) of the diversity detected by microscopy, which counted 65 ciliate morphotypes. The most representative classes in both approaches were Spirotrichea and Oligohymenophorea. The metabarcoding analysis revealed that 35.3% of the ciliate MOTUs had less than 97% similarity to available sequences in the NCBI database, indicating that more than one-third of these MOTUs potentially represents still not represented or undescribed ciliate species in current databases. Our findings indicate that metabarcoding techniques can significantly enhance the comprehension of ciliate diversity in tropical environments, but the scarcity of reference sequences of brackish ciliates in molecular databases represents a challenge to the taxonomic assignment of the MOTUs. This study provides new insights into the diversity of ciliates in a threatened coastal lagoon, revealing a vast array of still unknown and rare ciliate taxonomic units in tropical environments.
Keywords: Brackish water; Brazil, Rio de Janeiro; Ciliophora; Coastal lagoon; Eukaryotes; Metabarcoding; Microscopy.
© 2024. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors state they have no conflicts of interest in this submission.
Figures





Similar articles
-
Exploring the impact of urban pollution on ciliate diversity along the Sapucaí River (Minas Gerais, Brazil) via DNA metabarcoding.Mol Biol Rep. 2024 Sep 9;51(1):967. doi: 10.1007/s11033-024-09908-5. Mol Biol Rep. 2024. PMID: 39249572
-
Ciliate diversity and distribution patterns in the sediments of a seamount and adjacent abyssal plains in the tropical Western Pacific Ocean.BMC Microbiol. 2017 Sep 12;17(1):192. doi: 10.1186/s12866-017-1103-6. BMC Microbiol. 2017. PMID: 28899339 Free PMC article.
-
Ciliate Diversity From Aquatic Environments in the Brazilian Atlantic Forest as Revealed by High-Throughput DNA Sequencing.Microb Ecol. 2021 Apr;81(3):630-643. doi: 10.1007/s00248-020-01612-8. Epub 2020 Oct 6. Microb Ecol. 2021. PMID: 33025060
-
Diversity of free-living marine ciliates (Alveolata, Ciliophora): Faunal studies in coastal waters of China during the years 2011-2016.Eur J Protistol. 2017 Oct;61(Pt B):424-438. doi: 10.1016/j.ejop.2017.04.007. Epub 2017 Apr 21. Eur J Protistol. 2017. PMID: 28545996 Review.
-
Updating Biodiversity Studies in Loricate Protists: The Case of the Tintinnids (Alveolata, Ciliophora, Spirotrichea).J Eukaryot Microbiol. 2016 Sep;63(5):651-6. doi: 10.1111/jeu.12303. Epub 2016 Mar 2. J Eukaryot Microbiol. 2016. PMID: 26863912 Review.
Cited by
-
Exploring the impact of urban pollution on ciliate diversity along the Sapucaí River (Minas Gerais, Brazil) via DNA metabarcoding.Mol Biol Rep. 2024 Sep 9;51(1):967. doi: 10.1007/s11033-024-09908-5. Mol Biol Rep. 2024. PMID: 39249572
-
A Reassessment of Phylogenetic Relationships in Class Oligohymenophorea (Protista, Ciliophora) Based on Updated Multigene Data.Ecol Evol. 2025 Feb 24;15(2):e70950. doi: 10.1002/ece3.70950. eCollection 2025 Feb. Ecol Evol. 2025. PMID: 40008061 Free PMC article.
-
Effect of applying oyster shell powder on soil properties and microbial diversity in the acidified soils of pomelo garden.Environ Microbiome. 2025 May 24;20(1):57. doi: 10.1186/s40793-025-00721-6. Environ Microbiome. 2025. PMID: 40413554 Free PMC article.
References
-
- Finlay BJ, Fenchel T. Ecology: Role of ciliates in the natural environment. In: Hausmann K, Bradbury PC, editors. Ciliates: Cells as Organisms. Germany: Gustav Fischer Verlag; 1996. pp. 417–440.
-
- Ritter CD, Machado AF, Ribeiro KF, Dunthorn M (2021) Metabarcoding advances for ecology and biogeography of neotropical protists: What do we know, where do we go? Biota Neotrop 21(4):e20211214. 10.1590/1676-0611-BN-2021-1214
-
- Campello-Nunes PH, Fernandes N, Schlegel M, Silva-Neto ID. Description and phylogenetic position of Corlissina maricaensis gen. nov., sp. nov. (Karyorelictea, Geleiidae), a novel interstitial ciliate from Brazil, with redefinition of the family Geleiidae. Int J Syst Evol Microbiol. 2015;65:4297–4308. doi: 10.1099/ijsem.0.000579. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

