Paraptotic Cell Death as an Unprecedented Mode of Action Observed for New Bipyridine-Silver(I) Compounds Bearing Phosphane Coligands
- PMID: 38401050
- PMCID: PMC11056982
- DOI: 10.1021/acs.jmedchem.3c01036
Paraptotic Cell Death as an Unprecedented Mode of Action Observed for New Bipyridine-Silver(I) Compounds Bearing Phosphane Coligands
Abstract
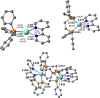
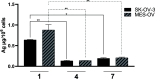
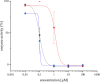
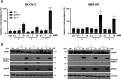
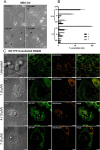
In this work, we investigated the anticancer activity of several novel silver(I) 2,2'-bipyridine complexes containing either triphenylphosphane (PPh3) or 1,2-bis(diphenylphosphino)ethane (dppe) ligands. All compounds were characterized by diverse analytical methods including ESI-MS spectrometry; NMR, UV-vis, and FTIR spectroscopies; and elemental analysis. Moreover, several compounds were also studied by X-ray single-crystal diffraction. Subsequently, the compounds were investigated for their anticancer activity against drug-resistant and -sensitive cancer cells. Noteworthily, neither carboplatin and oxaliplatin resistance nor p53 deletion impacted on their anticancer efficacy. MES-OV cells displayed exceptional hypersensitivity to the dppe-containing drugs. This effect was not based on thioredoxin reductase inhibition, enhanced drug uptake, or apoptosis induction. In contrast, dppe silver drugs induced paraptosis, a novel recently described form of programmed cell death. Together with the good tumor specificity of this compound's class, this work suggests that dppe-containing silver complexes could be interesting drug candidates for the treatment of resistant ovarian cancer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


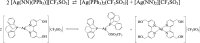





Similar articles
-
Improving Cytotoxicity against Breast Cancer Cells by Using Mixed-Ligand Ruthenium(II) Complexes of 2,2'-Bipyridine, Amino Acid, and Nitric Oxide Derivatives as Potential Anticancer Agents.Anticancer Agents Med Chem. 2021;21(12):1602-1611. doi: 10.2174/0929867327666201020155105. Anticancer Agents Med Chem. 2021. PMID: 33081686
-
Pd(II)/diphosphine/curcumin complexes as potential anticancer agents.Dalton Trans. 2024 Dec 3;53(47):18902-18916. doi: 10.1039/d4dt01045k. Dalton Trans. 2024. PMID: 38938129
-
Di- and polynuclear silver(I) saccharinate complexes of tertiary diphosphane ligands: synthesis, structures, in vitro DNA binding, and antibacterial and anticancer properties.J Biol Inorg Chem. 2014 Jan;19(1):29-44. doi: 10.1007/s00775-013-1052-y. Epub 2013 Oct 17. J Biol Inorg Chem. 2014. PMID: 24132752
-
Copper(II) complexes containing hydrazone and bipyridine/phenanthroline ligands for anticancer application against breast cancer cells.J Inorg Biochem. 2025 Jan;262:112759. doi: 10.1016/j.jinorgbio.2024.112759. Epub 2024 Oct 12. J Inorg Biochem. 2025. PMID: 39426333
-
A review on 1,1-bis(diphenylphosphino)methane bridged homo- and heterobimetallic complexes for anticancer applications: Synthesis, structure, and cytotoxicity.Eur J Med Chem. 2020 Oct 15;204:112613. doi: 10.1016/j.ejmech.2020.112613. Epub 2020 Jul 15. Eur J Med Chem. 2020. PMID: 32784095 Review.
Cited by
-
A green approach to improve antibacterial properties of PVC/PVDF film doped by silver nanoparticles via nanosecond laser ablation for wound healing application.Sci Rep. 2024 Nov 13;14(1):27926. doi: 10.1038/s41598-024-78841-1. Sci Rep. 2024. PMID: 39537795 Free PMC article.
-
Paraptosis-A Distinct Pathway to Cell Death.Int J Mol Sci. 2024 Oct 25;25(21):11478. doi: 10.3390/ijms252111478. Int J Mol Sci. 2024. PMID: 39519031 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

