Working memory signals in early visual cortex are present in weak and strong imagers
- PMID: 38401134
- PMCID: PMC10893972
- DOI: 10.1002/hbm.26590
Working memory signals in early visual cortex are present in weak and strong imagers
Abstract
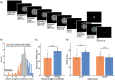
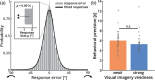
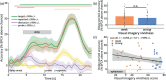
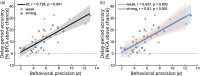
It has been suggested that visual images are memorized across brief periods of time by vividly imagining them as if they were still there. In line with this, the contents of both working memory and visual imagery are known to be encoded already in early visual cortex. If these signals in early visual areas were indeed to reflect a combined imagery and memory code, one would predict them to be weaker for individuals with reduced visual imagery vividness. Here, we systematically investigated this question in two groups of participants. Strong and weak imagers were asked to remember images across brief delay periods. We were able to reliably reconstruct the memorized stimuli from early visual cortex during the delay. Importantly, in contrast to the prediction, the quality of reconstruction was equally accurate for both strong and weak imagers. The decodable information also closely reflected behavioral precision in both groups, suggesting it could contribute to behavioral performance, even in the extreme case of completely aphantasic individuals. Our data thus suggest that working memory signals in early visual cortex can be present even in the (near) absence of phenomenal imagery.
Keywords: early visual cortex; individual differences; multivariate decoding; visual imagery; working memory.
© 2024 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Visual working memory in aphantasia: Retained accuracy and capacity with a different strategy.Cortex. 2021 Oct;143:237-253. doi: 10.1016/j.cortex.2021.07.012. Epub 2021 Aug 8. Cortex. 2021. PMID: 34482017
-
Aphantasia: The science of visual imagery extremes.Handb Clin Neurol. 2021;178:277-296. doi: 10.1016/B978-0-12-821377-3.00012-X. Handb Clin Neurol. 2021. PMID: 33832681
-
Shared representations for working memory and mental imagery in early visual cortex.Curr Biol. 2013 Aug 5;23(15):1427-31. doi: 10.1016/j.cub.2013.05.065. Epub 2013 Jul 18. Curr Biol. 2013. PMID: 23871239
-
The human imagination: the cognitive neuroscience of visual mental imagery.Nat Rev Neurosci. 2019 Oct;20(10):624-634. doi: 10.1038/s41583-019-0202-9. Nat Rev Neurosci. 2019. PMID: 31384033 Review.
-
Shared Neural Mechanisms of Visual Perception and Imagery.Trends Cogn Sci. 2019 May;23(5):423-434. doi: 10.1016/j.tics.2019.02.004. Epub 2019 Mar 12. Trends Cogn Sci. 2019. PMID: 30876729 Review.
Cited by
-
Uncovering the Role of the Early Visual Cortex in Visual Mental Imagery.Vision (Basel). 2024 May 2;8(2):29. doi: 10.3390/vision8020029. Vision (Basel). 2024. PMID: 38804350 Free PMC article. Review.
-
Neural dynamics of visual working memory representation during sensory distraction.Elife. 2025 Jun 24;13:RP99290. doi: 10.7554/eLife.99290. Elife. 2025. PMID: 40552615 Free PMC article.
-
Ocular working memory signals are flexible to behavioral priority and subjective imagery strength.J Neurophysiol. 2024 Jul 1;132(1):162-176. doi: 10.1152/jn.00446.2023. Epub 2024 Jun 5. J Neurophysiol. 2024. PMID: 38836298 Free PMC article.
-
The Neural Underpinnings of Aphantasia: A Case Study of Identical Twins.bioRxiv [Preprint]. 2025 Apr 24:2024.09.23.614521. doi: 10.1101/2024.09.23.614521. bioRxiv. 2025. Update in: Cereb Cortex. 2025 Jul 1;35(7):bhaf192. doi: 10.1093/cercor/bhaf192. PMID: 39386622 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

