Functional hypoxia reduces mitochondrial calcium uptake
- PMID: 38401291
- PMCID: PMC10906399
- DOI: 10.1016/j.redox.2024.103037
Functional hypoxia reduces mitochondrial calcium uptake
Abstract
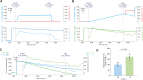
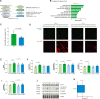
Mitochondrial respiration extends beyond ATP generation, with the organelle participating in many cellular and physiological processes. Parallel changes in components of the mitochondrial electron transfer system with respiration render it an appropriate hub for coordinating cellular adaption to changes in oxygen levels. How changes in respiration under functional hypoxia (i.e., when intracellular O2 levels limit mitochondrial respiration) are relayed by the electron transfer system to impact mitochondrial adaption and remodeling after hypoxic exposure remains poorly defined. This is largely due to challenges integrating findings under controlled and defined O2 levels in studies connecting functions of isolated mitochondria to humans during physical exercise. Here we present experiments under conditions of hypoxia in isolated mitochondria, myotubes and exercising humans. Performing steady-state respirometry with isolated mitochondria we found that oxygen limitation of respiration reduced electron flow and oxidative phosphorylation, lowered the mitochondrial membrane potential difference, and decreased mitochondrial calcium influx. Similarly, in myotubes under functional hypoxia mitochondrial calcium uptake decreased in response to sarcoplasmic reticulum calcium release for contraction. In both myotubes and human skeletal muscle this blunted mitochondrial adaptive responses and remodeling upon contractions. Our results suggest that by regulating calcium uptake the mitochondrial electron transfer system is a hub for coordinating cellular adaption under functional hypoxia.
Keywords: Coenzyme Q; Exercise; Membrane potential; Respirometry; Skeletal muscle.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest E.G. is the founder and CEO of Oroboros Instruments.
Figures



References
-
- Gnaiger E. Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis, 5th ed. Bioenerg. Commun. 2020;2020(2)
-
- Hermansen L., Saltin B. Oxygen uptake during maximal treadmill and bicycle exercise. J. Appl. Physiol. 1969;26(1):31–37. - PubMed
-
- Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001;128(3):277–297. - PubMed
-
- Donnelly C., et al. The ABC of hypoxia - what is the norm. Bioenerg. Commun. 2022;2022(12.v2)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

