Human papillomavirus negative high grade cervical lesions and cancers: Suggested guidance for HPV testing quality assurance
- PMID: 38401369
- PMCID: PMC11863830
- DOI: 10.1016/j.jcv.2024.105657
Human papillomavirus negative high grade cervical lesions and cancers: Suggested guidance for HPV testing quality assurance
Abstract
Background: Some high-grade cervical lesions and cervical cancers (HSIL+) test negative for human papillomavirus (HPV). The HPV-negative fraction varies between 0.03 % and 15 % between different laboratories. Monitoring and extended re-analysis of HPV-negative HSIL+ could thus be helpful to monitor performance of HPV testing services. We aimed to a) provide a real-life example of a quality assurance (QA) program based on re-analysis of HPV-negative HSIL+ and b) develop international guidance for QA of HPV testing services based on standardized identification of apparently HPV-negative HSIL+ and extended re-analysis, either by the primary laboratory or by a national HPV reference laboratory (NRL).
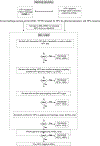
Methods: There were 116 initially HPV-negative cervical specimens (31 histopathology specimens and 85 liquid-based cytology samples) sent to the Swedish HPV Reference Laboratory for re-testing. Based on the results, an international QA guidance was developed through an iterative consensus process.
Result: Standard PCR testing detected HPV in 55.2 % (64/116) of initially "HPV-negative" samples. Whole genome sequencing of PCR-negative samples identified HPV in an additional 7 samples (overall 61.2 % HPV positivity). Reasons for failure to detect HPV in an HSIL+ lesion are listed and guidance to identify cases for extended re-testing, including which information should be included when referring samples to an NRL are presented.
Conclusion: Monitoring the proportion of and reasons for failure to detect HPV in HSIL+ will help support high performance and quality improvement of HPV testing services. We encourage implementation of QA strategies based on re-analysis of "HPV negative" HSIL+ samples.
Keywords: Cervical cancer; HPV testing; HSIL; Human papillomavirus; Quality assurance; Screening.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: K Cuschieri's institution has received research funding or gratis consumables to support research from the following commercial entities in the last 3 years: Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Abbott, Hologic and Vaccitech. M.P.’s institution received research funding, free-of-charge reagents, and consumables to support research in the last 3 years from Qiagen, Seegene, Abbott, and Roche, all paid to his employer. Sciensano the employer of M.A. received funding in the framework of Valgent and VALHUDES, which are 2 researcher induced protocols for evaluation of HPV tests on cervical and vaginal samples respectively (see Arbyn et J Clin Virol 2016 & 2018). M.A, did not receive any financial or material benefit from these projects.
Figures
References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189: 12–9. - PubMed
-
- Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, Barretos Cancer H, Baylor College of M, Beckman Research Institute of City of H, Buck Institute for Research on A, Canada’s Michael Smith Genome Sciences C, Harvard Medical S, Helen FGCC, Research Institute at Christiana Care Health S, HudsonAlpha Institute for B, et al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543: 378–84. - PMC - PubMed
-
- Fernandes A, Viveros-Carreno D, Hoegl J, Avila M, Pareja R. Human papillomavirus-independent cervical cancer. Int J Gynecol Cancer 2022;32: 1–7. - PubMed