Adjuvanted nanoliposomes displaying six hemagglutinins and neuraminidases as an influenza virus vaccine
- PMID: 38401547
- PMCID: PMC10982964
- DOI: 10.1016/j.xcrm.2024.101433
Adjuvanted nanoliposomes displaying six hemagglutinins and neuraminidases as an influenza virus vaccine
Abstract
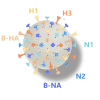
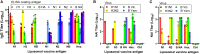
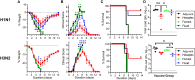
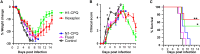
Inclusion of defined quantities of the two major surface proteins of influenza virus, hemagglutinin (HA) and neuraminidase (NA), could benefit seasonal influenza vaccines. Recombinant HA and NA multimeric proteins derived from three influenza serotypes, H1N1, H3N2, and type B, are surface displayed on nanoliposomes co-loaded with immunostimulatory adjuvants, generating "hexaplex" particles that are used to immunize mice. Protective immune responses to hexaplex liposomes involve functional antibody elicitation against each included antigen, comparable to vaccination with monovalent antigen particles. When compared to contemporary recombinant or adjuvanted influenza virus vaccines, hexaplex liposomes perform favorably in many areas, including antibody production, T cell activation, protection from lethal virus challenge, and protection following passive sera transfer. Based on these results, hexaplex liposomes warrant further investigation as an adjuvanted recombinant influenza vaccine formulation.
Keywords: adjuvant; antigen display; influenza virus; liposome; subunit vaccine.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.F.L. and W.-C.H. hold competing interests in POP Biotechnologies. W.-C.H. and H.L.K. are employed by POP Biotechnologies. Patents related to this work have been filed by the State University of New York.
Figures



Similar articles
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Cationic lipid/DNA complex-adjuvanted influenza A virus vaccination induces robust cross-protective immunity.J Virol. 2010 Dec;84(24):12691-702. doi: 10.1128/JVI.00769-10. Epub 2010 Oct 13. J Virol. 2010. PMID: 20943978 Free PMC article.
-
Extending the Stalk Enhances Immunogenicity of the Influenza Virus Neuraminidase.J Virol. 2019 Aug 28;93(18):e00840-19. doi: 10.1128/JVI.00840-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31375573 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
Cited by
-
Vaccine Strategies Against RNA Viruses: Current Advances and Future Directions.Vaccines (Basel). 2024 Nov 28;12(12):1345. doi: 10.3390/vaccines12121345. Vaccines (Basel). 2024. PMID: 39772007 Free PMC article. Review.
-
Sustained exposure to multivalent antigen-decorated nanoparticles generates broad anti-coronavirus responses.Matter. 2025 Apr 2;8(4):102006. doi: 10.1016/j.matt.2025.102006. Epub 2025 Feb 25. Matter. 2025. PMID: 40821443
-
Drug-phospholipid conjugate nano-assembly for drug delivery.Smart Med. 2024 Dec 22;3(4):e20240053. doi: 10.1002/SMMD.20240053. eCollection 2024 Dec. Smart Med. 2024. PMID: 39776594 Free PMC article. Review.
-
Nanocarriers for intracellular delivery of proteins in biomedical applications: strategies and recent advances.J Nanobiotechnology. 2024 Nov 10;22(1):688. doi: 10.1186/s12951-024-02969-5. J Nanobiotechnology. 2024. PMID: 39523313 Free PMC article. Review.
-
Genetically Predicted Peripheral Immune Cells Mediate the Effect of Gut Microbiota on Influenza Susceptibility.Int J Mol Sci. 2024 Jul 14;25(14):7706. doi: 10.3390/ijms25147706. Int J Mol Sci. 2024. PMID: 39062949 Free PMC article.
References
-
- Centers for Disease Control and Prevention . 2023. 2022-2023 U.S. Flu Season: Preliminary In-Season Burden Estimates.https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
-
- Gasparini R., Amicizia D., Lai P.L., Rossi S., Panatto D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V ® and Fluad ® ) in preventing hospitalization for influenza and pneumonia in the elderly: a matched case-control study. Hum. Vaccin. Immunother. 2013;9:144–152. doi: 10.4161/hv.22231. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

