Paediatric, maternal, and congenital mpox: a systematic review and meta-analysis
- PMID: 38401556
- PMCID: PMC11519316
- DOI: 10.1016/S2214-109X(23)00607-1
Paediatric, maternal, and congenital mpox: a systematic review and meta-analysis
Abstract
Background: Although mpox has been detected in paediatric populations in central and west Africa for decades, evidence synthesis on paediatric, maternal, and congenital mpox, and the use of vaccines and therapeutics in these groups, is lacking. A systematic review is therefore indicated to set the research agenda.
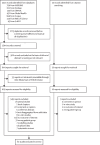
Methods: We conducted a systematic review and meta-analysis, searching articles in Embase, Global Health, MEDLINE, CINAHL, Web of Science, Scopus, SciELO, and WHO databases from inception to April 17, 2023. We included studies reporting primary data on at least one case of confirmed, suspected, or probable paediatric, maternal, or congenital mpox in humans or the use of third-generation smallpox or mpox vaccines, targeted antivirals, or immune therapies in at least one case in our population of interest. We included clinical trials and observational studies in humans and excluded reviews, commentaries, and grey literature. A pooled estimate of the paediatric case fatality ratio was obtained using random-effects meta-analysis. This study is registered with PROSPERO (CRD420223336648).
Findings: Of the 61 studies, 53 reported paediatric outcomes (n=2123 cases), seven reported maternal or congenital outcomes (n=32 cases), two reported vaccine safety (n=28 recipients), and three reported transmission during breastfeeding (n=4 cases). While a subset of seven observational studies (21 children and 12 pregnant individuals) reported uneventful treatment with tecovirimat, there were no randomised trials reporting safety or efficacy for any therapeutic agent. Among children, the commonest clinical features included rash (86 [100%] of 86), fever (63 [73%] of 86), and lymphadenopathy (40 [47%] of 86). Among pregnant individuals, rash was reported in 23 (100%) of 23; fever and lymphadenopathy were less common (six [26%] and three [13%] of 23, respectively). Most paediatric complications (12 [60%] of 20) arose from secondary bacterial infections. The pooled paediatric case fatality ratio was 11% (95% CI 4-20), I2=75%. Data from 12 pregnancies showed half resulted in fetal death. Research on vaccine and immune globulin safety remains scarce for children and absent for pregnant individuals.
Interpretation: Our review highlights critical knowledge gaps in the epidemiology, prevention, and treatment of mpox in children and pregnant individuals, especially those residing in endemic countries. Increased funding, international collaboration, and equitable research is needed to inform mpox control strategies tailored for at-risk communities in endemic countries.
Funding: None.
Translations: For the French, Spanish and Portuguese translations of the abstract see Supplementary Materials section.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SBD has received honoraria from MSD and Sanofi for taking part in respiratory syncytial virus (RSV) advisory boards and has provided consultancy and/or investigator roles in relation to product development for Janssen, AstraZeneca, Pfizer, Moderna, Valneva, MSD, iLiAD, and Sanofi with fees paid to St George's, University of London. SBD is a member of the UK Department of Health and Social Care's (DHSC) Joint Committee on Vaccination and Immunisation (JCVI) RSV subcommittee and Medicines and Healthcare products Regulatory Agency's (MHRA) Paediatric Medicine Expert Advisory Group (PMEAG), but the reviews expressed herein do not necessarily represent those of DHSC, JCVI, MHRA, or PMEAG. All other authors declare no competing interests.
Figures



Comment in
-
Ending the neglect of paediatric, maternal, and congenital mpox.Lancet Glob Health. 2024 Apr;12(4):e533-e534. doi: 10.1016/S2214-109X(24)00054-8. Epub 2024 Feb 21. Lancet Glob Health. 2024. PMID: 38401555 No abstract available.
References
-
- Whitehouse ER, Bonwitt J, Hughes CM, et al. Clinical and epidemiological findings from enhanced monkeypox surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011–2015. J Infect Dis. 2021;223:1870–1878. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

