Triple-fusion protein (TriFu): A potent, targeted, enzyme-like inhibitor of all three complement activation pathways
- PMID: 38401844
- PMCID: PMC11065761
- DOI: 10.1016/j.jbc.2024.105784
Triple-fusion protein (TriFu): A potent, targeted, enzyme-like inhibitor of all three complement activation pathways
Abstract
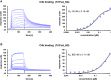
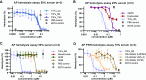
The introduction of a therapeutic anti-C5 antibody into clinical practice in 2007 inspired a surge into the development of complement-targeted therapies. This has led to the recent approval of a C3 inhibitory peptide, an antibody directed against C1s and a full pipeline of several complement inhibitors in preclinical and clinical development. However, no inhibitor is available that efficiently inhibits all three complement initiation pathways and targets host cell surface markers as well as complement opsonins. To overcome this, we engineered a novel fusion protein combining selected domains of the three natural complement regulatory proteins decay accelerating factor, factor H and complement receptor 1. Such a triple fusion complement inhibitor (TriFu) was recombinantly expressed and purified alongside multiple variants and its building blocks. We analyzed these proteins for ligand binding affinity and decay acceleration activity by surface plasmon resonance. Additionally, we tested complement inhibition in several in vitro/ex vivo assays using standard classical and alternative pathway restricted hemolysis assays next to hemolysis assays with paroxysmal nocturnal hemoglobinuria erythrocytes. A novel in vitro model of the alternative pathway disease C3 glomerulopathy was established to evaluate the potential of the inhibitors to stop C3 deposition on endothelial cells. Next to the novel engineered triple fusion variants which inactivate complement convertases in an enzyme-like fashion, stoichiometric complement inhibitors targeting C3, C5, factor B, and factor D were tested as comparators. The triple fusion approach yielded a potent complement inhibitor that efficiently inhibits all three complement initiation pathways while targeting to surface markers.
Keywords: complement; complement receptor 1; compstatin; decay accelerating factor; eculizumab; factor H; fusion protein; paroxysmal nocturnal hemoglobinuria.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest C. Q. S., H. S., B. H., A. D. and M. H.-L. are inventors of (a) patent application(s) that describes the use of complement inhibitors for therapeutic applications. C. Q. S. has received research funding from pharmaceutical companies. M. H.-L., C. Q. S., B. H. and H. S. received honoraria for speaking at symposia organized by Alexion Pharmaceuticals. H. S. served on advisory committees for Alexion AstraZeneca Rare Diseases, Sanofi, Sobi, Novartis, Amgen and received research funding from Alexion Pharmaceuticals (all to the University of Ulm). The other authors have disclosed no relevant conflict of interest with the contents of the article. B. K. G. and R. B. were/are employees at Takeda Pharmaceuticals.
Figures




References
-
- Hillmen P., Young N.S., Schubert J., Brodsky R.A., Socié G., Muus P., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006;355:1233–1243. - PubMed
-
- Legendre C.M., Licht C., Muus P., Greenbaum L.A., Babu S., Bedrosian C., et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2013;368:2169–2181. - PubMed
-
- Howard J.F., Utsugisawa K., Benatar M., Murai H., Barohn R.J., Illa I., et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986. - PubMed
-
- Pittock S.J., Berthele A., Fujihara K., Kim H.J., Levy M., Palace J., et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381:614–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

