Self-regulated reversal deformation and locomotion of structurally homogenous hydrogels subjected to constant light illumination
- PMID: 38402204
- PMCID: PMC10894256
- DOI: 10.1038/s41467-024-46100-6
Self-regulated reversal deformation and locomotion of structurally homogenous hydrogels subjected to constant light illumination
Abstract
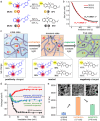
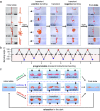
Environmentally adaptive hydrogels that are capable of reconfiguration in response to external stimuli have shown great potential toward bioinspired actuation and soft robotics. Previous efforts have focused mainly on either the sophisticated design of heterogeneously structured hydrogels or the complex manipulation of external stimuli, and achieving self-regulated reversal shape deformation in homogenous hydrogels under a constant stimulus has been challenging. Here, we report the molecular design of structurally homogenous hydrogels containing simultaneously two spiropyrans that exhibit self-regulated transient deformation reversal when subjected to constant illumination. The deformation reversal mechanism originates from the molecular sequential descending-ascending charge variation of two coexisting spiropyrans upon irradiation, resulting in a macroscale volumetric contraction-expansion of the hydrogels. Hydrogel film actuators were developed to display complex temporary bidirectional shape transformations and self-regulated reversal rolling under constant illumination. Our work represents an innovative strategy for programming complex shape transformations of homogeneous hydrogels using a single constant stimulus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Synchronizing Multicolor Changes and Shape Deformation Into Structurally Homogeneous Hydrogels via a Single Photochromophore.Adv Mater. 2025 Apr;37(16):e2500857. doi: 10.1002/adma.202500857. Epub 2025 Mar 10. Adv Mater. 2025. PMID: 40059611
-
Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions.Acc Chem Res. 2022 Jun 7;55(11):1533-1545. doi: 10.1021/acs.accounts.2c00046. Epub 2022 Apr 12. Acc Chem Res. 2022. PMID: 35413187
-
Shape-Memory Hydrogels: Evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks.Acc Chem Res. 2017 Apr 18;50(4):723-732. doi: 10.1021/acs.accounts.6b00584. Epub 2017 Feb 15. Acc Chem Res. 2017. PMID: 28199083
-
Recent progress in the shape deformation of polymeric hydrogels from memory to actuation.Chem Sci. 2021 Mar 24;12(19):6472-6487. doi: 10.1039/d0sc07106d. Chem Sci. 2021. PMID: 34040724 Free PMC article. Review.
-
Nanocomposite hydrogel actuators hybridized with various dimensional nanomaterials for stimuli responsiveness enhancement.Nano Converg. 2019 Jun 10;6(1):18. doi: 10.1186/s40580-019-0188-z. Nano Converg. 2019. PMID: 31179510 Free PMC article. Review.
Cited by
-
In Situ Stimuli Transfer in Multi-Environment Shape-Morphing Hydrogels Based on the Copolymer Between Spiropyran and Acrylic Acid.Adv Sci (Weinh). 2025 May;12(17):e2416173. doi: 10.1002/advs.202416173. Epub 2025 Mar 7. Adv Sci (Weinh). 2025. PMID: 40051303 Free PMC article.
-
Light-controlled smart materials: Supramolecular regulation and applications.Smart Mol. 2024 Sep 15;2(4):e20240036. doi: 10.1002/smo.20240036. eCollection 2024 Dec. Smart Mol. 2024. PMID: 40626274 Free PMC article. Review.
-
Solvent-adaptive hydrogels with lamellar confinement cellular structure for programmable multimodal locomotion.Nat Commun. 2024 Oct 26;15(1):9254. doi: 10.1038/s41467-024-53549-y. Nat Commun. 2024. PMID: 39461965 Free PMC article.
-
Theoretical Modeling of a Bionic Arm with Elastomer Fiber as Artificial Muscle Controlled by Periodic Illumination.Polymers (Basel). 2025 Jul 31;17(15):2122. doi: 10.3390/polym17152122. Polymers (Basel). 2025. PMID: 40808170 Free PMC article.
-
Self-Oscillation of Liquid Crystal Elastomer Fiber-Slide System Driven by Self-Flickering Light Source.Polymers (Basel). 2024 Nov 26;16(23):3298. doi: 10.3390/polym16233298. Polymers (Basel). 2024. PMID: 39684043 Free PMC article.
References
-
- Dawson J, Vincent JFV, Rocca AM. How pine cones open. Nature. 1997;390:668–668. doi: 10.1038/37745. - DOI
-
- Oliver K, Seddon A, Trask RS. Morphing in nature and beyond: a review of natural and synthetic shape-changing materials and mechanisms. J. Mater. Sci. 2016;51:10663–10689. doi: 10.1007/s10853-016-0295-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

