Prior flavivirus immunity skews the yellow fever vaccine response to cross-reactive antibodies with potential to enhance dengue virus infection
- PMID: 38402207
- PMCID: PMC10894228
- DOI: 10.1038/s41467-024-45806-x
Prior flavivirus immunity skews the yellow fever vaccine response to cross-reactive antibodies with potential to enhance dengue virus infection
Abstract
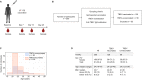
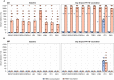
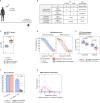
The yellow fever 17D vaccine (YF17D) is highly effective but is frequently administered to individuals with pre-existing cross-reactive immunity, potentially impacting their immune responses. Here, we investigate the impact of pre-existing flavivirus immunity induced by the tick-borne encephalitis virus (TBEV) vaccine on the response to YF17D vaccination in 250 individuals up to 28 days post-vaccination (pv) and 22 individuals sampled one-year pv. Our findings indicate that previous TBEV vaccination does not affect the early IgM-driven neutralizing response to YF17D. However, pre-vaccination sera enhance YF17D virus infection in vitro via antibody-dependent enhancement (ADE). Following YF17D vaccination, TBEV-pre-vaccinated individuals develop high amounts of cross-reactive IgG antibodies with poor neutralizing capacity. In contrast, TBEV-unvaccinated individuals elicit a non-cross-reacting neutralizing response. Using YF17D envelope protein mutants displaying different epitopes, we identify quaternary dimeric epitopes as the primary target of neutralizing antibodies. Additionally, TBEV-pre-vaccination skews the IgG response towards the pan-flavivirus fusion loop epitope (FLE), capable of mediating ADE of dengue and Zika virus infections in vitro. Together, we propose that YF17D vaccination conceals the FLE in individuals without prior flavivirus exposure but favors a cross-reactive IgG response in TBEV-pre-vaccinated recipients directed to the FLE with potential to enhance dengue virus infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. This work was supported by FlavImmunity a combined grant of the German Research foundation (DFG) project number 391217598 to SR and ABK and the French National Research Agency (ANR) project number ANR-17-CE15-0031-01 to GBS; by DFG TRR237 grant project number 369799452 to SR and ABK (TRR237 TPB14) and BMK (TRR237 TPA04); by Yellow4Flavi a horizon Europe grant project number 101137459 funded by the European Union to SR, AK GBS, KS; by grants of the iMed consortium of the German Helmholtz Societies to SR; by the Einheit für Klinische Pharmakologie (EKLIP), Helmholtz Zentrum München, Neuherberg, Germany to SR and SE; KS and EMS are supported by the BMBF, project 01KI2013; a Stipend (TI 07.003) by the German Center for Infection Research (DZIF) to FL; grants by the Friedrich Baur Foundation (FBS) to JTS, HK and MP; a Metiphys fellowship of the Medical Faculty of the LMU Munich to MP; by the FöFoLe Program of the Medical Faculty of the LMU Munich to SG, EN, LL, FL, the international doctoral program “iTarget: Immunotargeting of cancer” funded by the Elite Network of Bavaria to ASP, MZ, SG, LL, EN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures




Similar articles
-
Impact of flavivirus vaccine-induced immunity on primary Zika virus antibody response in humans.PLoS Negl Trop Dis. 2020 Feb 4;14(2):e0008034. doi: 10.1371/journal.pntd.0008034. eCollection 2020 Feb. PLoS Negl Trop Dis. 2020. PMID: 32017766 Free PMC article.
-
Effect of previous heterologous flavivirus vaccinations on human antibody responses in tick-borne encephalitis and dengue virus infections.J Med Virol. 2023 Nov;95(11):e29245. doi: 10.1002/jmv.29245. J Med Virol. 2023. PMID: 38009693 Free PMC article.
-
Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in adults primed with a Japanese encephalitis virus or yellow fever virus vaccine in the USA: a phase 1, randomised, double-blind, placebo-controlled clinical trial.Lancet Infect Dis. 2023 Oct;23(10):1175-1185. doi: 10.1016/S1473-3099(23)00192-5. Epub 2023 Jun 27. Lancet Infect Dis. 2023. PMID: 37390836 Free PMC article. Clinical Trial.
-
Cross-reactive dengue virus-derived monoclonal antibodies to Zika virus envelope protein: Panacea or Pandora's box?BMC Infect Dis. 2018 Dec 10;18(1):641. doi: 10.1186/s12879-018-3572-0. BMC Infect Dis. 2018. PMID: 30526531 Free PMC article. Review.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
Cited by
-
Factors determining the outcomes of immune imprinting after repeated orthoflavivirus infections.Front Immunol. 2025 Jul 16;16:1560851. doi: 10.3389/fimmu.2025.1560851. eCollection 2025. Front Immunol. 2025. PMID: 40740782 Free PMC article. Review.
-
Zika virus-specific and orthoflavivirus-cross-reactive IgGs correlate with Zika virus seroneutralization depending on prior dengue virus infection.PLoS Negl Trop Dis. 2025 Jul 9;19(7):e0013274. doi: 10.1371/journal.pntd.0013274. eCollection 2025 Jul. PLoS Negl Trop Dis. 2025. PMID: 40632785 Free PMC article.
-
Toward a deeper understanding of dengue: novel method for quantification and isolation of envelope protein epitope-specific antibodies.mSphere. 2025 May 27;10(5):e0096124. doi: 10.1128/msphere.00961-24. Epub 2025 Apr 11. mSphere. 2025. PMID: 40214258 Free PMC article.
-
Antigen-specific T cell responses following single and co-administration of tick-borne encephalitis, Japanese encephalitis, and yellow fever virus vaccines: Results from an open-label, non-randomized clinical trial-cohort.PLoS Negl Trop Dis. 2025 Feb 28;19(2):e0012693. doi: 10.1371/journal.pntd.0012693. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 40019865 Free PMC article. Clinical Trial.
-
Proceedings of the second annual dengue endgame summit: A call to action.PLoS Negl Trop Dis. 2025 Apr 28;19(4):e0013028. doi: 10.1371/journal.pntd.0013028. eCollection 2025 Apr. PLoS Negl Trop Dis. 2025. PMID: 40294026 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 391217598/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 369799452/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 391217598/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 369799452/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 101137459/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- ANR-17-CE15-0031-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-17-CE15-0031-01/Agence Nationale de la Recherche (French National Research Agency)
- 01KI2013/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01KI2013/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Medical

