T-DOpE probes reveal sensitivity of hippocampal oscillations to cannabinoids in behaving mice
- PMID: 38402238
- PMCID: PMC10894268
- DOI: 10.1038/s41467-024-46021-4
T-DOpE probes reveal sensitivity of hippocampal oscillations to cannabinoids in behaving mice
Abstract
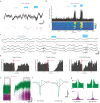
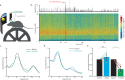
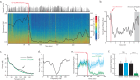
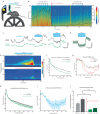
Understanding the neural basis of behavior requires monitoring and manipulating combinations of physiological elements and their interactions in behaving animals. We developed a thermal tapering process enabling fabrication of low-cost, flexible probes combining ultrafine features: dense electrodes, optical waveguides, and microfluidic channels. Furthermore, we developed a semi-automated backend connection allowing scalable assembly. We demonstrate T-DOpE (Tapered Drug delivery, Optical stimulation, and Electrophysiology) probes achieve in single neuron-scale devices (1) high-fidelity electrophysiological recording (2) focal drug delivery and (3) optical stimulation. The device tip can be miniaturized (as small as 50 µm) to minimize tissue damage while the ~20 times larger backend allows for industrial-scale connectorization. T-DOpE probes implanted in mouse hippocampus revealed canonical neuronal activity at the level of local field potentials (LFP) and neural spiking. Taking advantage of the triple-functionality of these probes, we monitored LFP while manipulating cannabinoid receptors (CB1R; microfluidic agonist delivery) and CA1 neuronal activity (optogenetics). Focal infusion of CB1R agonist downregulated theta and sharp wave-ripple oscillations (SPW-Rs). Furthermore, we found that CB1R activation reduces sharp wave-ripples by impairing the innate SPW-R-generating ability of the CA1 circuit.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Tapered Drug delivery, Optical stimulation, and Electrophysiology (T-DOpE) probes reveal the importance of cannabinoid signaling in hippocampal CA1 oscillations in behaving mice.bioRxiv [Preprint]. 2023 Jun 9:2023.06.08.544251. doi: 10.1101/2023.06.08.544251. bioRxiv. 2023. PMID: 37333172 Free PMC article. Preprint.
-
Routing of Hippocampal Ripples to Subcortical Structures via the Lateral Septum.Neuron. 2020 Jan 8;105(1):138-149.e5. doi: 10.1016/j.neuron.2019.10.012. Epub 2019 Nov 26. Neuron. 2020. PMID: 31784288 Free PMC article.
-
The Firing of Theta State-Related Septal Cholinergic Neurons Disrupt Hippocampal Ripple Oscillations via Muscarinic Receptors.J Neurosci. 2020 Apr 29;40(18):3591-3603. doi: 10.1523/JNEUROSCI.1568-19.2020. Epub 2020 Apr 7. J Neurosci. 2020. PMID: 32265261 Free PMC article.
-
Multifunctional Fibers as Tools for Neuroscience and Neuroengineering.Acc Chem Res. 2018 Apr 17;51(4):829-838. doi: 10.1021/acs.accounts.7b00558. Epub 2018 Mar 21. Acc Chem Res. 2018. PMID: 29561583 Review.
-
Exploring Ripple Waves in the Human Brain.Clin EEG Neurosci. 2023 Nov;54(6):594-600. doi: 10.1177/15500594211034371. Epub 2021 Jul 21. Clin EEG Neurosci. 2023. PMID: 34287087 Review.
Cited by
-
Flexible multimaterial fibers in modern biomedical applications.Natl Sci Rev. 2024 Sep 23;11(10):nwae333. doi: 10.1093/nsr/nwae333. eCollection 2024 Oct. Natl Sci Rev. 2024. PMID: 39411353 Free PMC article. Review.
-
Thermally drawn multi-material fibers: from fundamental research to industrial applications.Natl Sci Rev. 2024 Aug 24;11(10):nwae290. doi: 10.1093/nsr/nwae290. eCollection 2024 Oct. Natl Sci Rev. 2024. PMID: 39301079 Free PMC article.
-
Multifunctional Tetrode-like Drug delivery, Optical stimulation, and Electrophysiology (Tetro-DOpE) probes.Biosens Bioelectron. 2024 Dec 1;265:116696. doi: 10.1016/j.bios.2024.116696. Epub 2024 Aug 23. Biosens Bioelectron. 2024. PMID: 39208508
-
Differential contributions of CA3 and entorhinal cortex inputs to ripple patterns in the hippocampus.iScience. 2025 Jan 10;28(2):111782. doi: 10.1016/j.isci.2025.111782. eCollection 2025 Feb 21. iScience. 2025. PMID: 39967864 Free PMC article.
References
-
- Ko, E., Vöröslakos, M., Buzsáki, G. & Yoon, E. flexLiTE: flexible micro-LED integrated optoelectrodes for minimally-invasive chronic deep-brain study. bioRxiv, 10.1101/2022.08.05.503006 (2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

