Mosaic quadrivalent influenza vaccine single nanoparticle characterization
- PMID: 38402303
- PMCID: PMC10894272
- DOI: 10.1038/s41598-024-54876-2
Mosaic quadrivalent influenza vaccine single nanoparticle characterization
Abstract
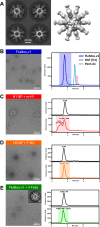
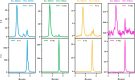
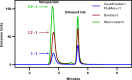
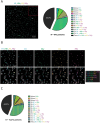
Recent work by our laboratory and others indicates that co-display of multiple antigens on protein-based nanoparticles may be key to induce cross-reactive antibodies that provide broad protection against disease. To reach the ultimate goal of a universal vaccine for seasonal influenza, a mosaic influenza nanoparticle vaccine (FluMos-v1) was developed for clinical trial (NCT04896086). FluMos-v1 is unique in that it is designed to co-display four recently circulating haemagglutinin (HA) strains; however, current vaccine analysis techniques are limited to nanoparticle population analysis, thus, are unable to determine the valency of an individual nanoparticle. For the first time, we demonstrate by total internal reflection fluorescence microscopy and supportive physical-chemical methods that the co-display of four antigens is indeed achieved in single nanoparticles. Additionally, we have determined percentages of multivalent (mosaic) nanoparticles with four, three, or two HA proteins. The integrated imaging and physicochemical methods we have developed for single nanoparticle multivalency will serve to further understand immunogenicity data from our current FluMos-v1 clinical trial.
Keywords: ELISA; Fluorescence imaging; Fluorescent labeling; Influenza vaccine; Mass spectrometry; Nanoparticle; Size-exclusion chromatography; TIRFM.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Development and validation of a mass spectrometry based analytical method to quantify the ratios in hemagglutinin trimers in quadrivalent influenza nanoparticle vaccine - FluMos-v1.Anal Methods. 2023 Feb 16;15(7):896-900. doi: 10.1039/d2ay01890j. Anal Methods. 2023. PMID: 36723411
-
Quantitation of strain-specific hemagglutinin trimers in mosaic quadrivalent influenza nanoparticle vaccine by ELISA.Vaccine. 2023 Aug 7;41(35):5201-5210. doi: 10.1016/j.vaccine.2023.07.009. Epub 2023 Jul 12. Vaccine. 2023. PMID: 37451877
-
Development of Influenza B Universal Vaccine Candidates Using the "Mosaic" Hemagglutinin Approach.J Virol. 2019 May 29;93(12):e00333-19. doi: 10.1128/JVI.00333-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944178 Free PMC article.
-
Towards a universal influenza vaccine: different approaches for one goal.Virol J. 2018 Jan 19;15(1):17. doi: 10.1186/s12985-017-0918-y. Virol J. 2018. PMID: 29370862 Free PMC article. Review.
-
A Perspective on Nanoparticle Universal Influenza Vaccines.ACS Infect Dis. 2018 Dec 14;4(12):1656-1665. doi: 10.1021/acsinfecdis.8b00206. Epub 2018 Nov 15. ACS Infect Dis. 2018. PMID: 30394725 Free PMC article. Review.
Cited by
-
SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses.Vaccines (Basel). 2025 Jul 22;13(8):778. doi: 10.3390/vaccines13080778. Vaccines (Basel). 2025. PMID: 40872865 Free PMC article.
-
Vaccination against influenza viruses annually: Renewing or narrowing the protective shield?J Exp Med. 2025 Jul 7;222(7):e20241283. doi: 10.1084/jem.20241283. Epub 2025 Apr 24. J Exp Med. 2025. PMID: 40272481 Review.
-
Synthesis of Chirally Chimeric Protein Nanoparticle Vaccines via Mirror-Image Spy Chemistry.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202509419. doi: 10.1002/anie.202509419. Epub 2025 Jun 25. Angew Chem Int Ed Engl. 2025. PMID: 40528680 Free PMC article.
-
Progress and Challenges in HIV-1 Vaccine Research: A Comprehensive Overview.Vaccines (Basel). 2025 Jan 31;13(2):148. doi: 10.3390/vaccines13020148. Vaccines (Basel). 2025. PMID: 40006695 Free PMC article. Review.
-
Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza.Vaccines (Basel). 2025 Jul 10;13(7):745. doi: 10.3390/vaccines13070745. Vaccines (Basel). 2025. PMID: 40733722 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

