Effects of sodium-glucose co-transporter 2 inhibitors on ultrafiltration in patients with peritoneal dialysis: a protocol for a randomized, double-blind, placebo-controlled, crossover trial (EMPOWERED)
- PMID: 38402502
- PMCID: PMC11189947
- DOI: 10.1007/s10157-024-02467-w
Effects of sodium-glucose co-transporter 2 inhibitors on ultrafiltration in patients with peritoneal dialysis: a protocol for a randomized, double-blind, placebo-controlled, crossover trial (EMPOWERED)
Abstract
Background: Volume overload is common and associated with high mortality in patients on peritoneal dialysis (PD). Traditional strategies including diuretics, water/salt restriction, and icodextrin-based solutions cannot always fully correct this condition, necessitating novel alternative strategies. Recent studies confirmed the expression of sodium-glucose cotransporter 2 (SGLT2) in the human peritoneum. Experimental data suggest that SGLT2 inhibitors decrease glucose absorption from the PD solution, thereby increasing the ultrafiltration volume. This trial aims to assess whether SGLT2 inhibitors increase the ultrafiltration volume in patients on PD.
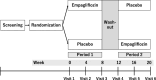
Methods: The EMPOWERED trial (trial registration: jRCTs051230081) is a multicenter, randomized, double-blind, placebo-controlled, crossover trial. Patients with clinically diagnosed chronic heart failure are eligible regardless of the presence of diabetes if they use at least 3 L/day glucose-based PD solutions. Participants will be randomly assigned (1:1) to receive empagliflozin 10 mg once daily and then placebo or vice versa. Each treatment period will last 8 weeks with a 4-week washout period. This study will recruit at least 36 randomized participants. The primary endpoint is the change in the daily ultrafiltration volume from baseline to week 8 in each intervention period. The key secondary endpoints include changes in the biomarkers of drained PD solutions, renal residual function, and anemia-related parameters.
Conclusions: This trial aims to assess the benefit of SGLT2 inhibitors in fluid management with a novel mechanism of action in patients on PD. It will also provide insights into the effects of SGLT2 inhibitors on solute transport across the peritoneal membrane and residual renal function.
Keywords: Peritoneal dialysis; SGLT2 inhibitor; Ultrafiltration; Volume overload.
© 2024. The Author(s).
Conflict of interest statement
Employment: Yumi Nakazono, Yoichi Nishiya (Nippon Boehringer Ingelheim Co., Ltd.), Consultancies: Yoshitaka Isaka (Nippon Boehringer Ingelheim Co., Ltd.), Honoraria: Yoshitaka Isaka (Nippon Boehringer Ingelheim Co., Ltd.). Other authors have no conflict of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources