A topological deep learning framework for neural spike decoding
- PMID: 38402607
- PMCID: PMC11393671
- DOI: 10.1016/j.bpj.2024.01.025
A topological deep learning framework for neural spike decoding
Abstract
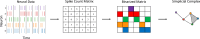
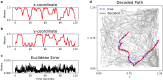
The brain's spatial orientation system uses different neuron ensembles to aid in environment-based navigation. Two of the ways brains encode spatial information are through head direction cells and grid cells. Brains use head direction cells to determine orientation, whereas grid cells consist of layers of decked neurons that overlay to provide environment-based navigation. These neurons fire in ensembles where several neurons fire at once to activate a single head direction or grid. We want to capture this firing structure and use it to decode head direction and animal location from head direction and grid cell activity. Understanding, representing, and decoding these neural structures require models that encompass higher-order connectivity, more than the one-dimensional connectivity that traditional graph-based models provide. To that end, in this work, we develop a topological deep learning framework for neural spike train decoding. Our framework combines unsupervised simplicial complex discovery with the power of deep learning via a new architecture we develop herein called a simplicial convolutional recurrent neural network. Simplicial complexes, topological spaces that use not only vertices and edges but also higher-dimensional objects, naturally generalize graphs and capture more than just pairwise relationships. Additionally, this approach does not require prior knowledge of the neural activity beyond spike counts, which removes the need for similarity measurements. The effectiveness and versatility of the simplicial convolutional neural network is demonstrated on head direction and trajectory prediction via head direction and grid cell datasets.
Copyright © 2024 Biophysical Society. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

