Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment
- PMID: 38402610
- PMCID: PMC11300849
- DOI: 10.1016/j.ccell.2024.01.013
Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment
Abstract
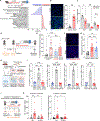
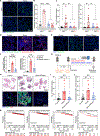
Chronic stress is associated with increased risk of metastasis and poor survival in cancer patients, yet the reasons are unclear. We show that chronic stress increases lung metastasis from disseminated cancer cells 2- to 4-fold in mice. Chronic stress significantly alters the lung microenvironment, with fibronectin accumulation, reduced T cell infiltration, and increased neutrophil infiltration. Depleting neutrophils abolishes stress-induced metastasis. Chronic stress shifts normal circadian rhythm of neutrophils and causes increased neutrophil extracellular trap (NET) formation via glucocorticoid release. In mice with neutrophil-specific glucocorticoid receptor deletion, chronic stress fails to increase NETs and metastasis. Furthermore, digesting NETs with DNase I prevents chronic stress-induced metastasis. Together, our data show that glucocorticoids released during chronic stress cause NET formation and establish a metastasis-promoting microenvironment. Therefore, NETs could be targets for preventing metastatic recurrence in cancer patients, many of whom will experience chronic stress due to their disease.
Keywords: breast cancer; chronic stress; glucocorticoids; metastasis; metastatic niche; neutrophil extracellular traps; tumor microenvironment; tumor-host interactions.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.E. serves on the advisory board of Cancer Cell. C.R.V. has received consulting fees from Flare Therapeutics, Roivant Sciences, and C4 Therapeutics; has served on the advisory boards of KSQ Therapeutics, Syros Pharmaceuticals, and Treeline Biosciences; and owns stock from Treeline Biosciences.
Figures


Comment in
-
Stressed out neutrophils drive metastasis.Immunity. 2024 Apr 9;57(4):840-842. doi: 10.1016/j.immuni.2024.03.013. Immunity. 2024. PMID: 38599176
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

